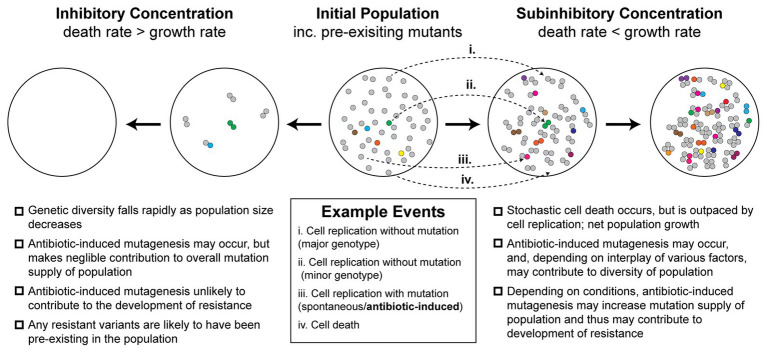
Figure 1.
Antibiotic-induced mutagenesis is one of many factors that influence genetic diversity in bacterial populations. Many antibiotics induce elevated rates of mutagenesis in bacterial cells. However, the relative importance of antibiotic-induced mutagenesis in shaping the evolution of antibiotic resistance remains unclear. This figure illustrates some of the competing factors that increase and decrease genetic diversity (i.e., number of unique mutants) in antibiotic-exposed bacteria. It is not intended to serve as a functional model of resistance development. An initial population of antibiotic-naïve cells appears in the center. The majority of cells in the population are of the major genotype (“wild type”; gray circles). Due to there being a basal rate of spontaneous mutagenesis, some cells have variant genotypes (colored circles). The left panels depict a scenario in which a lethal dose of bactericidal antibiotics is administered. Under these conditions, the rate of cell death outpaces the rate of cell division. In the first stage of this inhibitory concentration scenario, most of the population is killed (circles disappear). A few cells initially survive long enough to divide, however, in time these are also killed. Thus, no cells remain alive by the second stage of the scenario. Because there is little cell growth, there is little scope for antibiotic-induced mutagenesis. The genetic diversity of the population crashes as rapid cell death dominates over other factors. Any antibiotic-resistant mutants that are selected under these conditions are likely to be pre-existing in the population prior to antibiotic exposure. In the second scenario, depicted in the two right panels, a sub-inhibitory concentration of a bactericidal antibiotic is applied. As demonstrated recently (Coates et al., 2018), many cells die under these conditions, however, the rate of cell division outpaces the rate of cell death, leading to net population growth. In the first stage of this sub-inhibitory concentration scenario, a portion of cells die (~30%), while the remaining cells divide (~70%). Antibiotic-induced mutagenesis increases the probability that variants are produced during these cell divisions (gray circles more often yield colored circles). Specific examples of each event are depicted with arrows (i–iv; see example events legend). These processes of stochastic cell death, cell division, and mutagenesis are repeated in the second stage. Over time, the population becomes larger and contains a greater number of variants than the initial population. It is important to note, however, that the number of cell divisions required to reach a particular population size will be higher than what would be required in the absence of drug. Many experimental measures of induced mutagenesis rates neglect this behavior and will therefore be overestimated if cell death is occurring frequently (Frenoy and Bonhoeffer, 2018). Nevertheless, antibiotic-induced mutagenesis can significantly contribute to the development of antibiotic resistance in vivo (Cirz et al., 2005). Overall, the genetic diversity of the population in the sub-inhibitory concentration scenario will depend on the complex interplay between cell death, cell division, spontaneous mutagenesis, and induced mutagenesis. Selection for antibiotic-resistant phenotypes will occur at all stages and is likely to involve additional complexity arising from population-level phenomena that are not depicted in the figure.