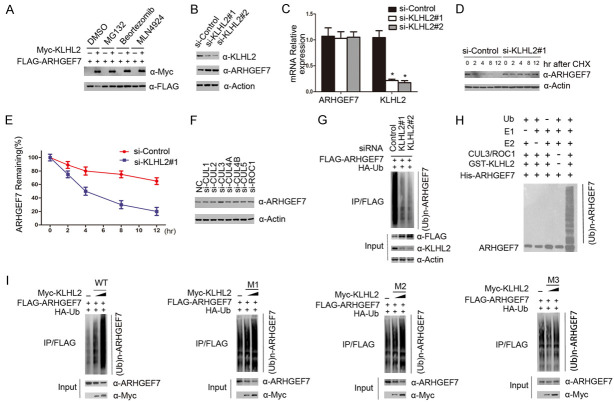
Figure 3.
The KLHL2-CUL3-ROC1 complex targets ARHGEF7 for ubiquitination. A. KLHL2 regulates ARHGEF7 protein level through the ubiquitin-proteasome pathway. 293T cells were transfected with FLAG-ARHGEF7 and increasing amounts of Myc-KLHL2 constructs. Twenty-four hours after transfection, cells were treated with 20 μM MG132, Bortezomib 100 mM, MLN4924, or DMSO for 6 h before cell lysates were prepared for WB analyses. B. Western blot of the indicated proteins in cell lysates from 786-O cells transfected with siRNAs and control. C. 786-O cells were transfected with the control or two independent KLHL2 siRNAs, respectively, 48 h after transfection, cells were harvested for qRT-PCR analysis. GAPDH was used for normalization. The mean values (S.D.) of three independent experiments are shown. D, E. KLHL2 knockdown prolonged the half-life of ARHGEF7 protein. 786-O cells were transfected with control or KLHL2 siRNA, 48 h after transfection, 786-O cells were treated with 30 μM cycloheximide for the indicated periods. Cells were harvested for Western blotting analyses. F. Western blot of indicated proteins in cell lysates from 786-O cells transfected with indicated siRNAs. G. KLHL2 was depleted by siRNAs in 293T cells transiently expressing HA-Ub and FLAG-ARHGEF7. FLAG-ARHGEF7 protein was immunoprecipitated and subjected to WB analyses. H. Western blot of the products of in vitro ubiquitination assay performed by incubating the reconstituted KLHL2-CUL3-ROC1 E3 ligase complex with E1, E2, Ub, and His-ARHGEF7 at 30°C for 2 h. I. The indicated plasmids were transfected into 293T cells. Twenty-four hours after transfection, cells were treated with 20 μM MG132 for 6 h. ARHGEF7 proteins were immunoprecipitated with anti-Flag antibody and analysed by WB.