Fig. 2.
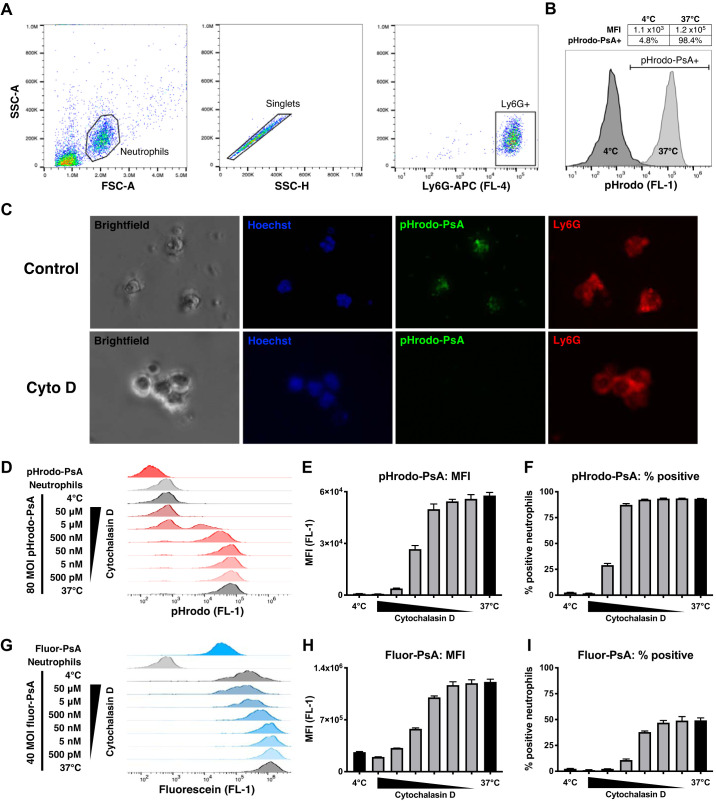
Ex vivo phagocytosis assay. A: flow cytometry gating strategy for identifying bronchoalveolar lavage (BAL) neutrophils. Mice were infected with 2 × 105 Pseudomonas aeruginosa (PsA) and euthanized 16 h later to obtain BAL. From the total BAL cell population, a neutrophil gate was selected using scatter properties [forward scatter (FSC), side scatter (SSC)], singlets were identified, and Ly6G+ cells were used for analysis. B: uptake of pHrodo-PsA by neutrophils. Compared with negative controls incubated at 4°C, samples at 37°C showed uptake of pHrodo-PsA as indicated by a shift in FL-1 fluorescence. Phagocytosis was quantified by mean fluorescence intensity (MFI) and by the percentage of neutrophils positive for pHrodo-PsA (% phagocytosis). C: fluorescence images demonstrating uptake of pHrodo-PsA by LPS-elicited Ly6G+ neutrophils (top). Phagocytosis was inhibited by 10 μM cytochalasin D (Cyto D; bottom). D: raw flow cytometry traces of pHrodo-PsA uptake by neutrophils. Fluorescence of pHrodo-PsA alone, neutrophils alone, and pHrodo-PsA plus neutrophils at 4°C was negligible. At 37°C, phagocytosis was dose-dependently inhibited by cytochalasin D. Quantification of pHrodo-PsA uptake by MFI (E) and % phagocytosis (F). G: raw flow cytometry traces of fluor-PsA uptake by neutrophils. Unphagocytosed fluor-PsA was highly fluorescent, leading to a strong signal in 4°C negative controls due to substrate adherence. At 37°C, phagocytosis was inhibited by cytochalasin D. Quantification of fluor-PsA uptake by MFI (H) and % phagocytosis (I) showed diminished dynamic range compared with pHrodo-PsA. Error bars represent SE. MOI, multiplicity of infection.