Table 3. Results of Selected Asymmetric FC Alkylation Reactions of Indoles with MTNSa.
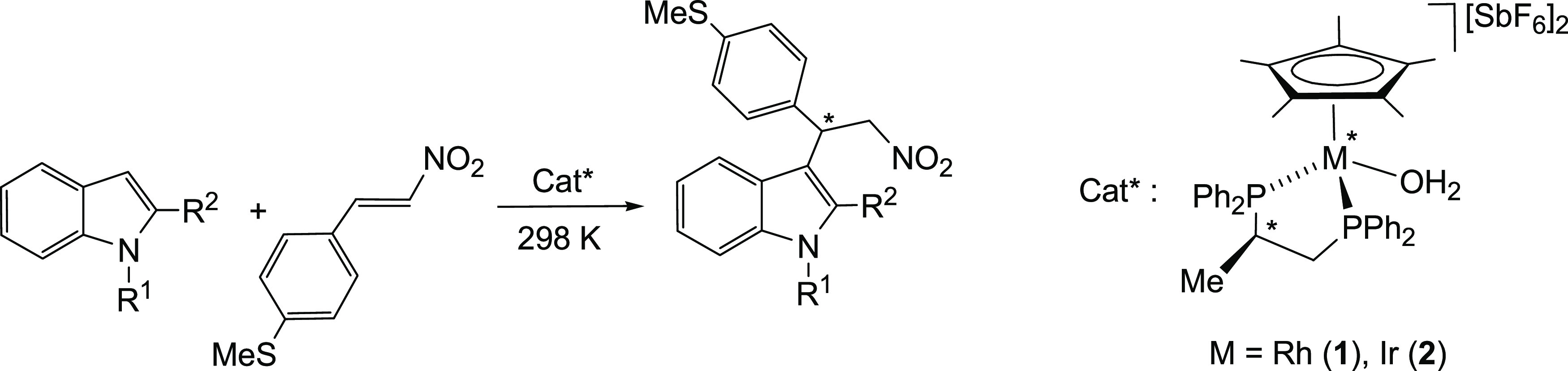
| entry | cat* | R1 | R2 | t (min) | conv.b (%) | eec (%) |
|---|---|---|---|---|---|---|
| 1 | 1 | H | H | 30 | 55 | rac |
| 2 | 2 | 30 | 22 | –2 | ||
| 3 | 1 | Me | H | 30 | 90 | +12 |
| 4 | 2 | 30 | 43 | +15 | ||
| 5 | 1 | H | Me | 30 | 81 | +3 |
| 6 | 2 | 30 | 48 | +15 | ||
| 7 | 1 | Me | Me | 5 | 100 | +93 |
| 8 | 2 | 30 | 100 | –21 |
Reagents and conditions: catalyst (0.03 mmol, 5.0 mol %), MTNS (0.90 mmol), indole (0.60 mmol), 4 Å molecular sieves (100 mg), and CH2Cl2 (4 mL).
Based on indole. Determined by 1H NMR spectroscopy.
Determined by high-performance liquid chromatography (HPLC). Changes in the sign indicate that the opposite enantiomer was preferentially obtained.
