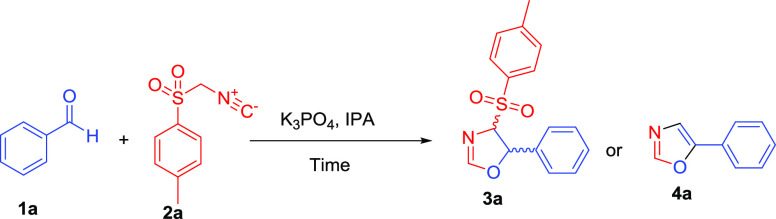
Table 3. Reaction Optimization Using K3PO4 Base Equivalentsa.
| entry | base | equiv | temperature (°C) | time (h) | oxazoline yield (%)b3a | oxazole yield (%)b4a |
|---|---|---|---|---|---|---|
| 1 | K3PO4 | 0.3 | 60 | 12 | 70 | 0 |
| 2 | K3PO4 | 0.5 | 60 | 12 | 85 | 0 |
| 3 | K3PO4 | 1.0 | 60 | 12 | 90 | 0 |
| 4 | K3PO4 | 1.5 | 60 | 12 | 65 | 30 |
| 5 | K3PO4 | 1 | MWc, 60 | 0.13 | 94 | 0 |
Reaction was performed using 1a (1.18 mmol) and 2a (1.18 mmol).
Yield of the isolated product.
Microwave reactions were carried out in a microwave model no. CATA R (Catalyst Systems, Pune) using a power of 280 W.