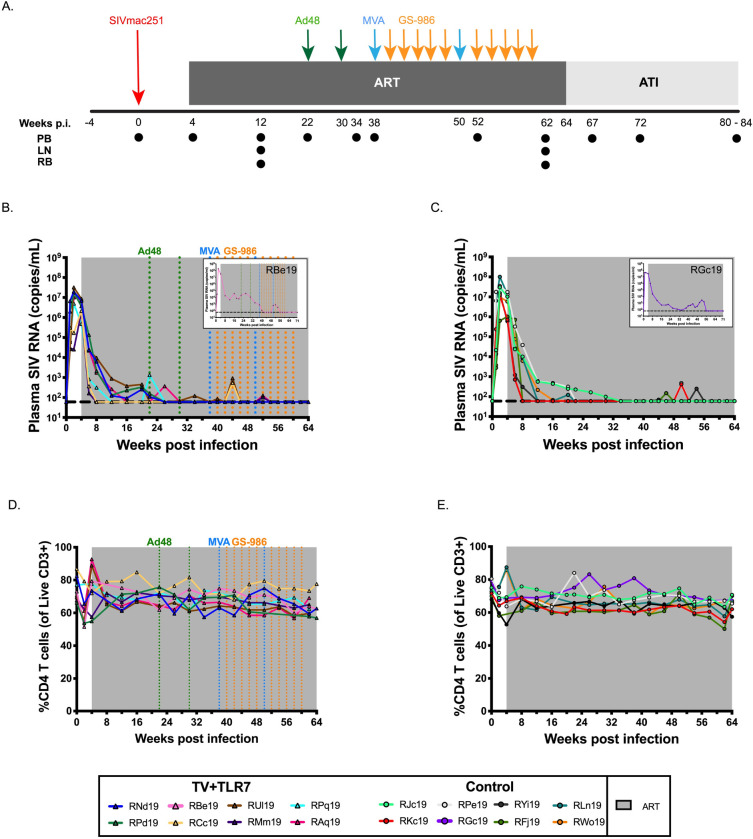
Fig 1. Experimental design and response to ART in SIV-infected infant RMs.
(A) Schematic of the study design. Sixteen infant RMs were infected orally with 105 TCID50 SIVmac251 (day 0), and starting on 4 weeks post infection (p.i.) treated with combination ART (TDF, FTC, DTG) for 15 months. Eight animals received 2 doses of Ad48-SIVsmE543 gag-pol-env (3x1010 viral particles, i.m.), 2 doses of MVA-SIVsmE543 gag-pol-env (1x108 PFU, i.m.), and 10 doses of GS-986 (0.3 mg/kg, o.g.), at the timepoints indicated (TV+TLR7). The remaining 8 animals served as ART-treated controls. At 64–71 weeks p.i. RMs underwent analytical treatment interruption (ATI) and all the animals were monitored for 4 to 6 months. PB, RB, and LN biopsies were collected at the indicated timepoints. Longitudinal analysis of plasma SIV RNA levels in (B) TV+TLR7 and (C) control RMs. The shaded area represents the period of ART treatment. The dashed line represents the limit of detection of the assay. Longitudinal analysis of peripheral CD4+ T cell frequency in (D) TV+TLR7 and (E) control RMs. The shaded area represents the period of ART treatment.