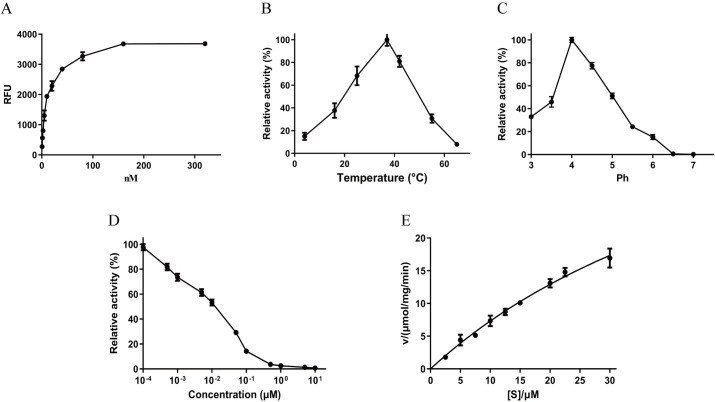
Fig 5. Biochemical properties of ySjCB2.
(A) Enzyme activity of ySjCB2 was determined by fluorescent substrate Z-FR-MCA under different enzyme concentrations (0.625, 1.25, 2.5, 5.0, 10.0, 20.0, 40.0, 80.0, 160.0 and 320.0 nM). (B) Optimal temperature at pH 4.0. Maximal activity was shown as 100%. (C) Optimal pH, enzyme activity was assayed in various pH buffers ranging from pH 3.0–8.0. Maximal activity was shown as 100%. (D) Inhibition profile for E-64 was determined by incubating ySjCB2 (50 nM) with different concentrations of E-64 in 100 mM acetic acid-sodium acetate (pH 4.0) buffer at room temperature for 30 min. Residual activities (%) were determined using Z-FR-MCA as a substrate. (E) The Michaelis–Menten curve, at 37°C, pH 4.0.