Figure 1.
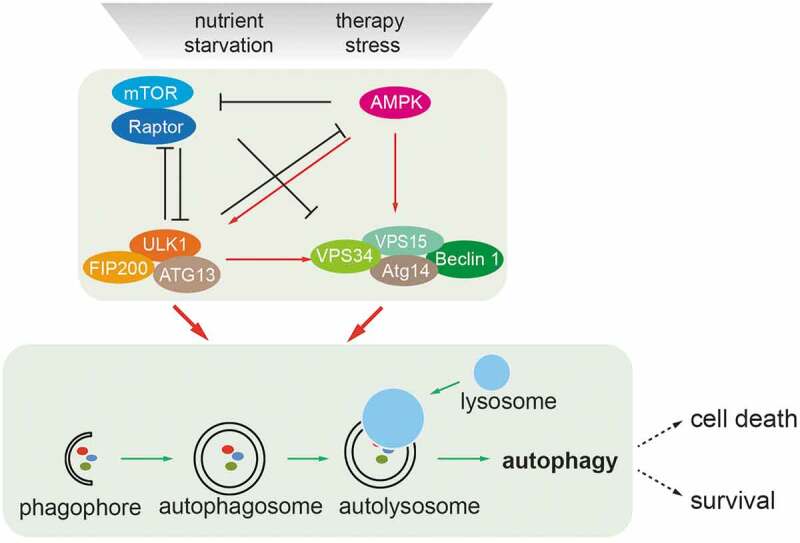
Nutrient-induced regulation of autophagy. Nutrient deprivation and therapy stress activate the metabolic sensors mTOR and AMP-activated kinase (AMPK) which act as major molecular switches of autophagy in response to nutrients. Under conditions of sufficient nutrients, mTOR complex 1 (mTORC1) is activated by upstream regulators, such as amino acid-sensing pathways. Active mTORC1 negatively regulates autophagy induction by phosphorylating and inactivating the ULK1 complex (ULK1/FIP200/ATG13) and the VPS34/BECN1complex (VPS34/BECN1/ATG14/VPS15). When nutrients are limiting, such as under glucose starvation, activated AMPK directly promotes autophagy by phosphorylating and activating the ULK1 complex and the VPS34/BECN1 complex. Activated AMPK and ULK1 negatively regulate mTORC1 activity, thereby relieving its inhibitory effect on autophagy. Autophagy starts from an isolation membrane (phagophore), followed by the formation of a double-membraned autophagosome, which engulfs intra-cellular proteins and subcellular organelles. Autophagosomes then mature by fusion with lysosomes, leading to the degradation of autophagosomal contents. Cells survive in the case of limited autophagy, such as in therapy-resistant cancer cells, whereas with high levels of autophagy cells will succumb to cell death
