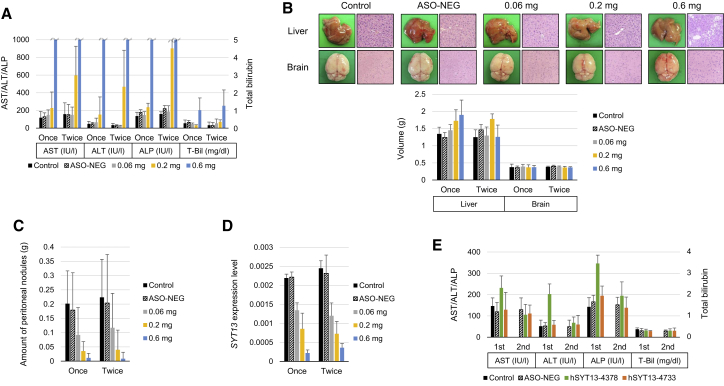
Figure 7.
Toxicity of Intraperitoneal Administration of AmNA-Modified Anti-SYT13 ASOs
(A) Liver function after treatment for 4 weeks. (B) Appearance (twice weekly groups), histological findings (twice weekly groups), and volumes of the liver and brain after treatment for 4 weeks. (C) Total volume of peritoneal nodules of each treatment group. (D) SYT13 mRNA levels in the peritoneal nodules. (E) Blood tests after 2 weeks administration of 0.2 mg hSYT13-4378 or hSYT13-4733, twice weekly and 2 weeks after treatment ceased. Error bars indicate the standard deviation.