Fig. 1: SLC38A9NT triggers FLCN GAP activity and disassembles the LFC.
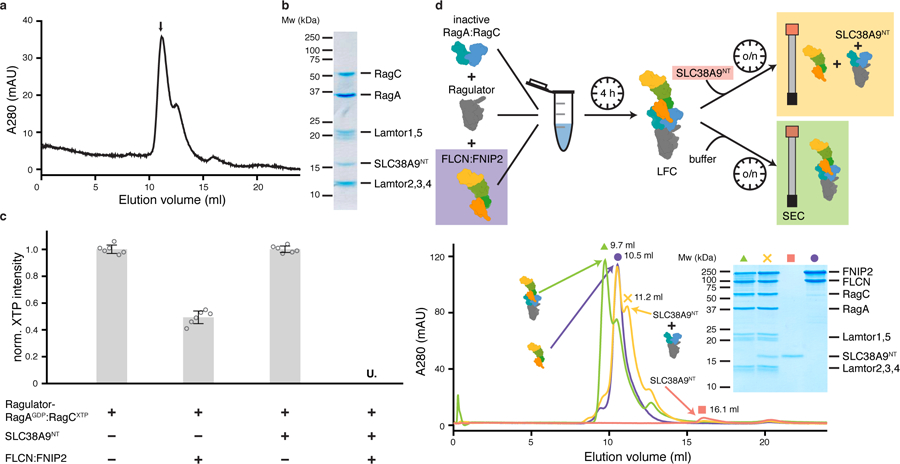
a, SEC profile of the reconstituted SLC38A9NT-RagAGDP:RagCXTPγS-Ragulator complex. XTPγS, non-hydrolyzable XTP analogue. b, SDS-PAGE analysis of the peak fraction indicated in a. c, HPLC-based RagC XTPase assay to measure FLCN GAP activity. Plotted are the mean ± standard deviation (SD) and individual data points of two independent experiments with technical triplicates each (n=6). U. means XTP signal was undetectable; norm., normalized; XTP, xanthosine tri-phosphate serving as a GTP analogue. d, LFC disassembly by SLC38A9NT shown by size exclusion chromatography (SEC). The experimental setup is illustrated (top) and the respective SEC traces and SDS-PAGE analysis it shown (bottom). Green (triangle), LFC; yellow (cross), LFC + SLC38A9NT; red (square), SLC38A9NT in isolation; purple (circle), FLCN:FNIP2 in isolation. Note that in the SDS-PAGE analysis, FLCN:FNIP2 can be detected in the same fraction as the pre-GAP complex because of peak overlap due to the very similar molecular weight of FLCN:FNIP2 and the pre-GAP complex (~187 kDa vs. ~170 kDa) (yellow cross). The presence of SLCN38A9NT in this fraction can only be explained by the fact that it is bound to Ragulator-Rag, as it elutes at 16.1 ml in isolation (red square). Uncropped gel images for panel b and d are shown in the Source Data. Data for graphs in a, c and d are available as source data online.
