Dear editor,
COVID‐19 pandemic continues to spread across the world in late August 2020. To date, total cases of COVID‐19 exceed 24.5 million including 833.556 deaths according to the WHO data. Governments still taking numerous measures to prevent the spread of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2). 1 The estimate for the basic reproduction number during the early stages of the COVID‐19 outbreak is recently reported as 4.22 ± 1.69 in Europe that shows COVID‐19 has a high transmission rate. In addition, the median incubation period is reported as 6 (minimum 2, maximum 14) days. 2 Although hydroxychloroquine, oseltamivir, remdesivir, favipiravir have been reported as an anti‐SARS‐CoV‐2 effect, it is still unclear the fully effective protective drug and treatment. 3
A 31‐year‐old man who had a high fever, chills, loss of smell, and the sore threat have presented to the pandemic hospital. He had close contact history with COVID‐19 patients in his workplace 6 days ago. The patient has been diagnosed with COVID‐19 based on the real‐time reverse‐transcriptase‐polymerase chain reaction (RT‐PCR) in nasopharyngeal swabs. He had been asked to quarantine at home and started orally 2x200 mg hydroxychloroquine. Although he had close contact with his wife without measure in the home his wife did not show any symptoms and signs of COVID‐19. Eighteen days after the first symptoms, the antibody test has been performed on the patient and his wife. The patient had IgG positivity while his wife did not develop antibody positivity including both IgM and IgG (Figure 1). She had been receiving Tenofovir 245 mg daily for 2 years for chronic hepatitis B. She did not have any other disease as well as drug history.
FIGURE 1.
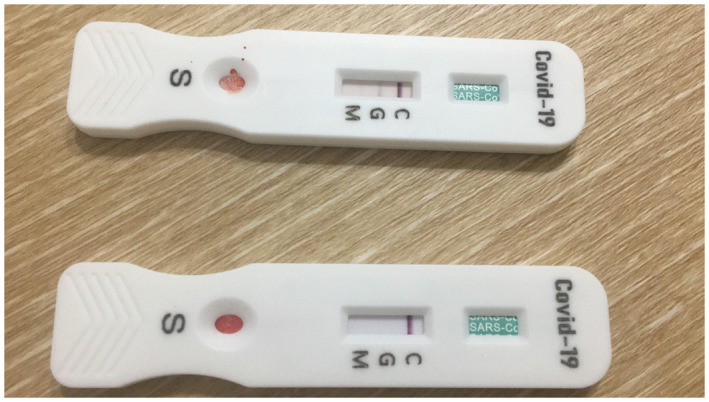
The patient who developed SARS‐CoV‐2 IgG antibody (above) and the test result of the patient's wife who receives Tenofovir (below) [Novacheck Diagnostic Kit (Colloidal Gold) for IgG/IgM antibody to SARS CoV‐2]
SARS‐CoV‐2 is a positive‐sense single‐strand RNA virus that has RNA‐dependent RNA polymerase property. 4 RNA‐dependent RNA polymerase is one of the main target points for the treatment of RNA viruses. Nucleotide analogs act as substrates and inhibit viral polymerases. Tenofovir diphosphate which is a nucleoside analog reverse‐transcriptase inhibitor is an active form of Tenofovir alafenamide that can be used for both hepatitis B and HIV/AIDS. 5 Recently, it has been shown that nucleoside analogs including 3′‐fluoro‐3′‐deoxythymidine triphosphate, 3′‐azido‐3′deoxythymidine triphosphate and Tenofovir diphosphate are able to terminate polymerase extension of coronavirus by consolidated with SARS‐CoV‐2 RNA‐dependent RNA polymerase. 6 Given the high reproduction number of SARS‐CoV‐2 and long‐term close contact of the case, it can be speculated that Tenofovir could interfere with the transmission of COVID‐19. In accordance with the previous in vitro study, our case is the first clinical report that supports the possible clinical protective effect of Tenofovir. Further randomised clinical studies will illuminate whether Tenofovir may be used as an effective new drug for COVID‐19.
DISCLOSURE
The author has declared no conflicts of interest for this article.
DATA AVAILABILITY STATEMENT
The data of this case are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.
REFERENCES
- 1. https://covid19.who.int/. Accessed August 29, 2020.
- 2. Linla K, Peirlinck M, Kuhl E. The reproduction number of COVID‐19 and its correlation with public health interventions. Comput Mech. 2020;28:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shyr ZA, Gorshkov K, Chen CZ, Zheng W. Drug discovery strategies for SARS‐CoV‐2. J Pharmacol Exp Ther. 2020;375:127‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zumla A, Chan JF, Azhar EI, Hui DS, Yuen KY. Coronaviruses‐drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Clercq E, Li G. Approved antiviral drugs over the past 50 years. Clin Microbiol Rev. 2016;29:695‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chien M, Anderson TK, Jockusch S, et al. Nucleotide analogues as inhibitors of SARS‐CoV‐2 polymerase, a Key Drug Target for COVID‐19. J Proteome Res. 2020;19(11):4690–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this case are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.


