Figure 1. Identification of sites within A1A2A3-VWF for cysteine-mediated PEGylation.
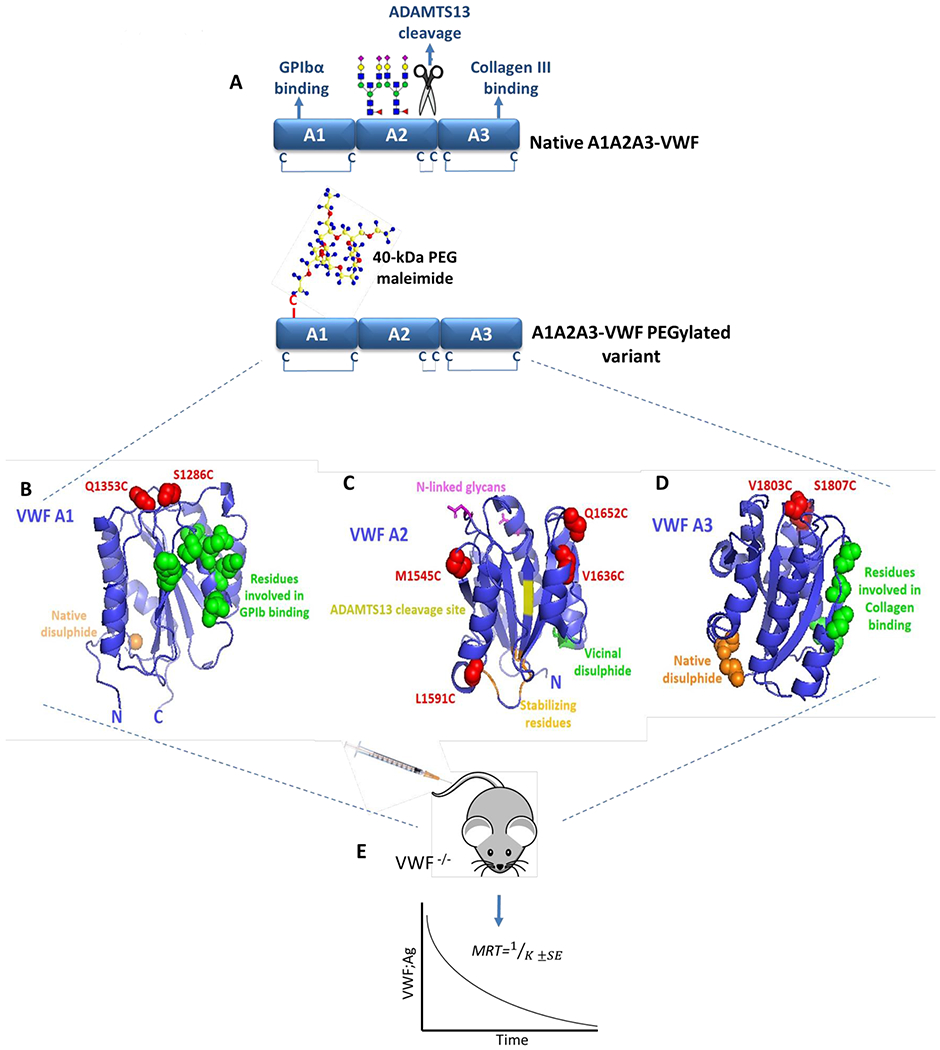
(A) Illustration of the tri-domain structure of native A1A2A3-VWF. GPIbα binding and type III collagen binding are regulated by specific sites within the A1- and A3-domains, respectively. In addition, the ADAMTS13 cleavage site and two N-linked glycan structures are located within the A2-domain. No free cysteine residues are present within A1A2A3-VWF, however a long-range disulphide bond exits within both the A1- and A3-domains while a vicinal disulphide bond is found within the C-terminal of the A2-domain. Specific sites within A1A2A3-VWF were selected to engineer novel free cysteine residues to subsequently facilitate covalent attachment of a 40-kDa PEG maleimide molecule. The crystal structure of A1 (B), A2 (C) and A3 (D) (Protein Data Bank ID, 1auq, 3zqk, 1ao3) are depicted with positions of novel engineered cysteine residues highlighted in red (S1286C and Q1353C in A1, M1545C, L1591C, V1636C and Q1652C in A2 and V1803C, S1807C in A3). (E) All novel A1A2A3-VWF variants were infused into VWF-deficient mice and the mean resident times (MRT) were calculated from the clearance rates.
