Figure 6. Site-specific N-linked glycosylation does not alter A1A2A3-VWF clearance or affinity for LRP1.
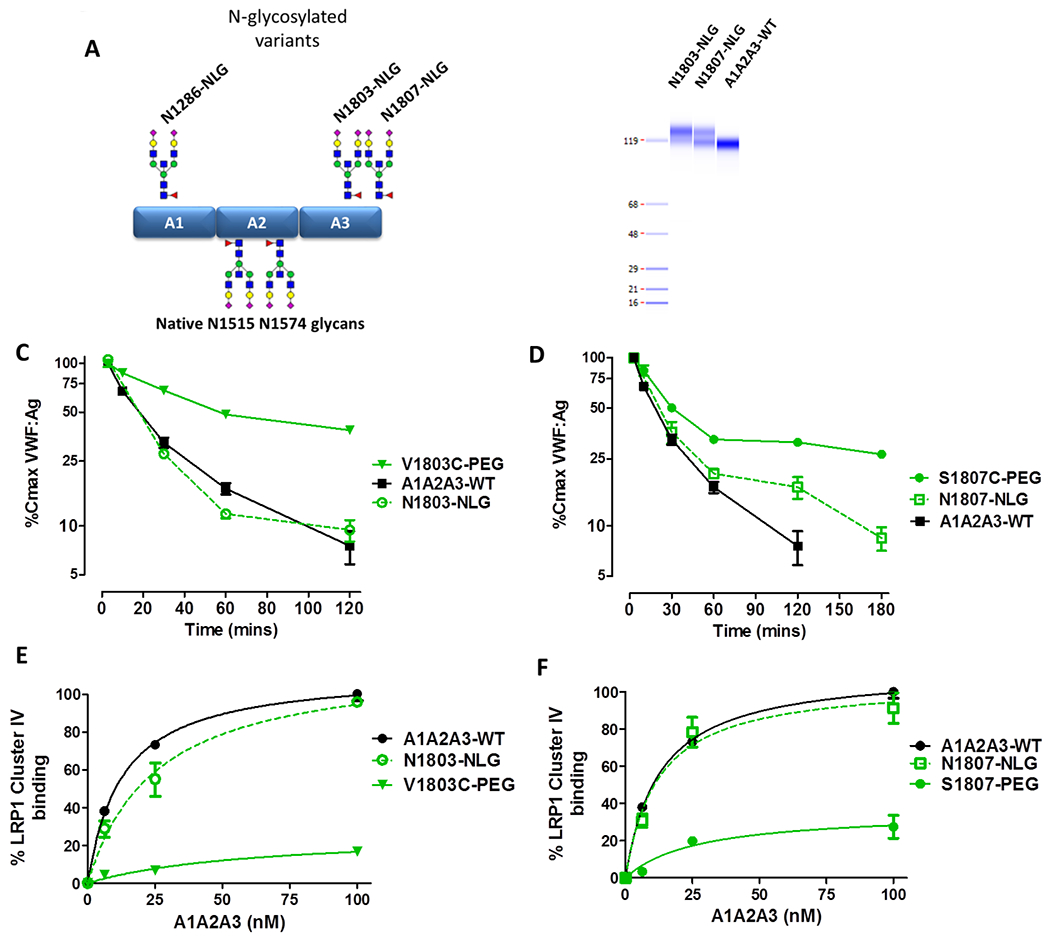
(A) Schematic illustration depicting the location of the three potential novel NLG sites with A1A2A3-VWF, N1286, N1803 and N1807. (B) N-linked occupancy was confirmed for N1803 and N1807 by capillary gel electrophoresis, as indicated by the presence of a higher molecular weight band. Clearance of N1803-NLG (C) and N1807-NLG (D) were assessed in VWF−/− mice. Immunosorbant assays were used to access binding to LRP1 cluster IV ligand binding domain for 1803-NLG (E) and 1807-NLG (F). All data is graphed as mean values ± SEM. For clearance studies, 3-5 mice per time point were used (* p <0.05, ** p <0.01, *** p <0.0001 respectively).
