Figure 4.
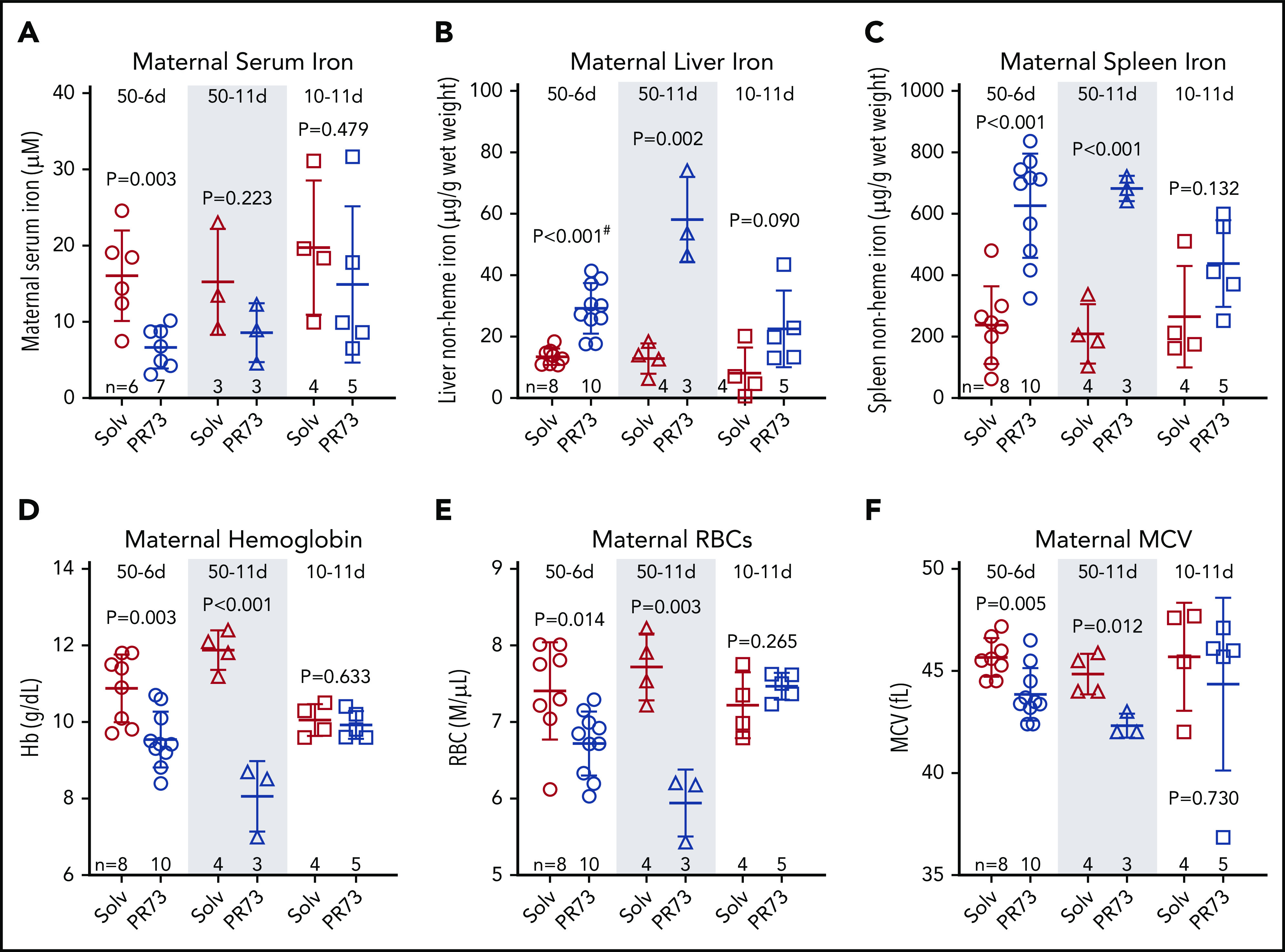
Effects of hepcidin-mediated iron restriction during pregnancy on maternal iron status and hematological parameters. Pregnant C57BL/6J dams received daily subcutaneous PR73 injections of 50 nmol/day for 6 days from E12.5 to E18.5 (50-6d), 50 nmol/day for 11 days from E7.5 to E17.5 (50-11d), 10 nmol/day for 11 days from E7.5 to E17.5 (10-11d) or the equivalent amount of solvent. Iron status and hematological parameters were measured at E18.5. Maternal iron parameters included serum iron (A), liver nonheme iron (B), and spleen nonheme iron (C). Maternal hematological parameters included hemoglobin (Hb) (D), RBC count (E), and MCV (F). Statistical comparisons were performed by 2-tailed Student t test for normally distributed values or for normally distributed but nonequal variance data sets, a 2-tailed t test with Welch’s correction was performed (indicated by # after the P value).
