Abstract
Objective
We sought to investigate whether there is any additional effect of coupled cognitive and physical rehabilitation compared to exercise training alone on walking and cognitive performance in individuals with relapsing remitting multiple sclerosis (RRMS).
Methods
A randomized controlled trial was conducted from March to November 2015 with 30 individuals with RRMS (aged 20 to 50 years; 21 women, 9 men), who underwent detailed medical and neurologic examination. They were randomly allocated using sealed envelopes to either the study group, who received physical and cognitive rehabilitation (dual-task training), or the control group, who received physical rehabilitation alone. Participants (in both groups) were assessed twice (8 weeks apart), before and after rehabilitation. Assessment tools were the Mini-Mental State Examination (MMSE), the Expanded Disability Status Scale (EDSS), neuropsychological evaluation (using RehaCom), and walking tests.
Results
After training, the control group significantly improved regarding MMSE, attention/concentration test, and 10-meter walking test, whereas the scores of the study group significantly improved in all studied parameters (Expanded Disability Status Scale, MMSE, logical reasoning, and attention/concentration and walking tests). The differential (delta) scores from before to after rehabilitation were significantly higher in the study group for logical reasoning, attention/concentration, and 2-minute walking distance scores.
Conclusions
Coupled physical and cognitive (dual-task) training showed concurrent improvement in cognitive and walking abilities in individuals with RRMS which exceeded that achieved by physical training alone.
Key Indexing Terms: Multiple Sclerosis, Cognitive and Physical Rehabilitation
Introduction
Multiple sclerosis (MS) is known as a chronic inflammatory autoimmune demyelinating disease of the central nervous system.1 There are 4 clinical types of MS, the most common of which is the relapsing remitting type. Approximately 87% of individuals with MS present with relapsing remitting multiple sclerosis (RRMS), characterized by acute attacks that evolve over days to weeks (relapses) followed by partial or full recovery over weeks to months (remission). In between attacks, the patient has no worsening of neurologic function.2 Individuals can manifest with different groups of neurologic symptoms including changes in vision, weakness, dyscoordination, sensory distortions or loss, or changes in bladder and bowel function. Less common but also disabling symptoms such as fatigue, cognitive change, and mood disturbance may occur in these individuals.3
MS prevalence has markedly risen in the last decade in the Middle-East.4 An estimated 70% of individuals with MS who have difficulty walking have rated it as the most frustrating aspect of MS.5 Gait has generally been viewed as an automated motor task that needs little higher level cognitive input. Recent studies, however, have proven that executive dysfunction and impaired attention are linked to gait disturbances.6 MS relapses may lead not only to residual motor disability but also to cognitive dysfunction. Among its features are deficits in complex attention, executive functioning, information processing efficiency, and long-term memory. These deficits by themselves and through their impact on gait notably affect the quality of life of individuals with MS.7 The available disease-modifying pharmacologic therapy has little impact on permanent motor and cognitive dysfunctions.8 Consequently, the management of these deficits relies on rehabilitation.9 A systematic review of 8 randomized controlled trials reports that combined cognitive and exercise training (delivered either sequentially or as dual-task training) can be efficacious for improving gait and cognitive outcomes in older adults who are healthy and cognitively impaired.10 Therefore, testing these rehabilitative strategies for individuals with MS is warranted. We hypothesized that the dual-task training (physical and cognitive) might improve cognitive and physical performance in comparison to physical rehabilitation only in individuals with RRMS. The aim of the current study, therefore, was to investigate the effect of coupled cognitive and physical (dual-task) rehabilitation compared to physical training alone on walking and cognitive performance in individuals with RRMS.
Methods
In this randomized controlled trial, individuals with RRMS were recruited from the Multiple Sclerosis Unit at Cairo University from March to November 2015. For the purposes of the study, the following inclusion criteria were defined:
-
•
A diagnosis of RRMS according to the McDonald criteria11
-
•
Age of 20 to 50 years
-
•
Expanded Disability Status Scale (EDSS)12 score of 4 to 6
-
•
Ability to cooperate and understand the instructions (requiring literacy and a minimum 6 years of primary education)
-
•
At least 3 months interval from the last relapse
Participants were excluded if, on clinical bases, they had other neurologic or psychiatric disorders (such as depression), had orthopedic or musculoskeletal problems (deformity in lower limbs or scoliosis), had profound visual or auditory dysfunction, had a relapse during the study, or did not attend the required number of rehabilitation sessions.
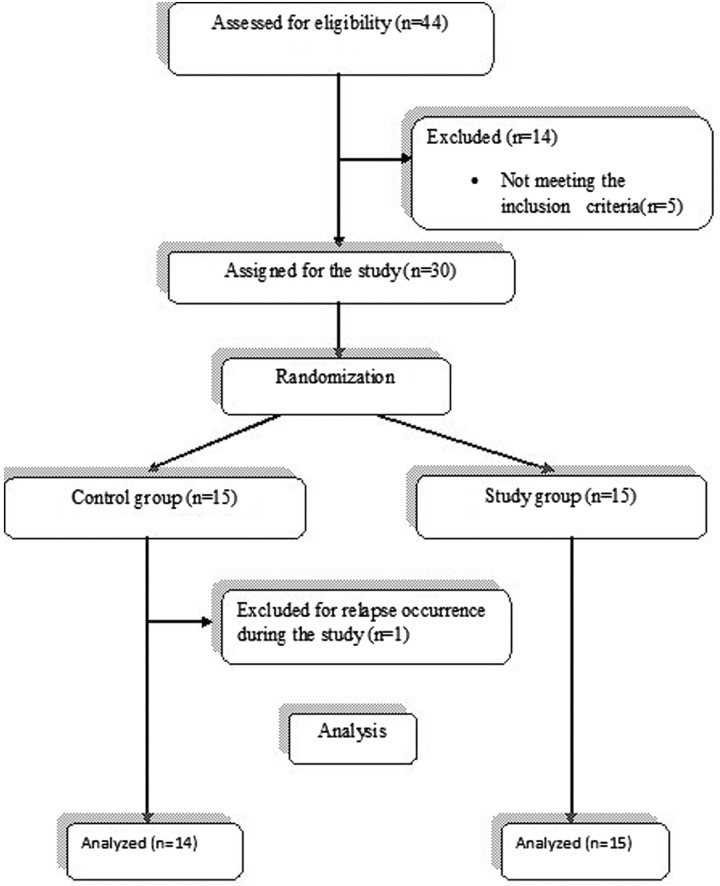
The study was approved by the ethical committee of the Faculty of Physical Therapy, Cairo University, Egypt. Forty-four individuals were assessed for eligibility; 5 did not meet the inclusion criteria and 9 declined to participate (1 due to husband refusal, 1 due to interference of work time and treatment sessions, and 7 due to personal problems precluding their regular attendance). Thus, 30 participants decided to complete the study: 9 men and 21 women. Participants were assigned randomly into 2 equal groups. However, because of the occurrence of a relapse, 1 of the participants in the control group was further excluded. Consequently, the control group included 14 patients and the study group included 15 (Fig 1). Participants were unaware of which intervention applied to them. Random assignment of participants was conducted by simple randomization using sealed envelopes. Consent was obtained from each participant after clarifying the study-related activities.
Fig 1.
Flowchart showing participation in the study.
Included participants underwent thorough medical and neurologic examination. The study group received motor and cognitive rehabilitation (dual-task training), and the control group received motor rehabilitation alone. Participants (in both groups) were assessed twice, before and after rehabilitation (8 weeks after the beginning of the rehabilitation program), by the same examiner. Assessment tools were the Mini-Mental State Examination (MMSE), the EDSS, neuropsychological evaluation using RehaCom, and walking assessments.
Mini-Mental State Examination
The MMSE was used to provide an indication of the general level of cognitive function.13
Expanded Disability Status Scale
The EDSS was used to quantify the severity of the disease.12
Neuropsychological Evaluation
This study was carried out using RehaCom software14 in the RehaCom lab of the Faculty of Physical Therapy, Cairo University. RehaCom (Hasomed, Magdeburg, Germany) is an intensive cognitive rehabilitation test that includes 32 assessment tasks for attention, logical reasoning, memory, and executive function. The procedure was performed through a regular personal computer with a 19-inch screen, RehaCom panel, and (1990-1997) EN/ISO-13485 certified software. For all tests, higher scores reflect better performance. Each performance and its progress can be saved on a hard drive. A set of neuropsychological tests was administered to each participant, including attention/concentration (A/C) and logical reasoning (LR) tests.
Attention/concentration tests have 100 levels of difficulty. Each level has an average of 22 subtests. The assessment starts at level 1 and progresses through the levels. A gray performance bar on the left side of the screen changes according to the participant's performance. It grows upward with correct answers and the participant progresses to a level of higher difficulty; shrinkage of the bar occurs with incorrect answers. More than 3 consecutive incorrect answers lead to test termination, and the participant's maximum level of achievement is recorded at the same level of difficulty. The session period was about 30 min.
Logical reasoning was assessed by a “completion of a series” exercise. The principle behind it is that the participant should learn to recognize the concepts underlying each problematic situation and use these concepts to solve the logic problem. In the assessment, a series of pictures is shown with simple graphic figures. When the participant has recognized what the rule is, he or she must then select the relevant picture from a matrix of pictures in the lower part of the screen. The series of 7 to 14 pictures appears in the upper part of the screen. If the number of pictures is greater than 7, the logical succession is distributed over 2 series or rows spaced out one above the other. A tear-off edge clarifies that the entire logical succession must be solved from the 2 individual series. When the correct picture is placed in the empty field, the picture series is solved. The field is beside a large red arrow. A performance column on the left-hand side of the screen increases with every correct selection. If in the assessment process the green marker is exceeded, the participant is considered as working well and a higher level of difficulty is set up with the next task. Switching to a lower level of difficulty occurs if the column does not reach the red marker. Otherwise, if the green marker has been reached but not exceeded, the same level of performance is repeated. The recorded session time was about 20 minutes.
Walking Assessments
Two walking assessments were administered:
-
•
Two-minute walking distance (2MWD) test. Participants were asked to walk for 2 minutes on a 40-meter-long course. The recommended length of the walking course is 30 meters (100 feet), but a range of 15 to 50 meters resulted in no significant difference.15 Participants were allowed to use their assistive devices (eg, a cane) while walking. They were instructed to dress comfortably and wear appropriate footwear. A comfortable ambient temperature was maintained throughout the test.
-
•
Ten-meter walking test (10mWT). This short walking test was conducted to investigate walking speed.
Rehabilitation Program
All the participants were trained 3 times a week for 8 weeks. Individualized training sessions took up to 60 minutes each. Physical rehabilitation for both groups of patients was in the form of postural and trunk control exercises, strengthening exercises, stretching and flexibility exercises, and balance and gait training. Each category of these exercises had a group of various graduations. In postural and trunk control, the participant stood with a normal wide base of support first steady in place (feet at the same level) and then with upper limb movement (forward, sideways, and upward), repeated several times with the eyes open (maintaining the same step) and then with eyes closed. For more difficult graduation, the participant stood with 1 foot in front of the other, foot-to-heel standing, step standing, and finally single limb support. The participant had to maintain for 30 seconds to consider it a completed level and had to repeat correctly 3 times to progress to the next level.
Gait training had many graduations: walking with walking aids, walking alone, walking forward, walking backward, walking with obstacle, walking on 1 line, tandem walking, walking with dorsiflexion, and finally walking on a balance board. Throughout the session, participants were allowed to take enough rest between exercises to overcome their fatigue.
Cognitive tasks added to the physical rehabilitation (dual-task training) for the study group were simple counting down16 from 100; continuous subtraction by 3; continuous subtraction by 7; naming as many animals or words as possible in 1 minute starting with a predefined letter, to be recalled later at the end of the session for memory training; asking simple questions; spelling words forward and backward; a visuospatial planning task (“How can you move from here to the reception?”); integrating language with calculation (“If Sunday is the 8th, what date will the following Thursday will be?”); naming objects; and remembering numbers.17 When a participant got tired, he or she was instructed to stop the motor task and focus on the cognitive task; this is called variable priority training.18
Statistical Analysis
The following steps were taken to determine the sample size and the power analysis using G*Power:
-
•
Due to a lack of previous studies in this regard and the inability to calculate the effect size, we conducted a pilot study for a sample of 10 patients.
-
•
The statistical analysis test (2 × 2 mixed-design multivariate analysis of variance) was conducted for this sample. SPSS revealed a Pillai trace of 0.4 that was used to detect the effect size using G*Power.
-
•
Power analysis (using G*Power version 3.0.10) revealed that 28 participants were sufficient to produce a power level of 83% with a detected effect size of 0.67.
-
•
Finally, we tested a larger sample (29 participants) to obtain a higher power level and greater effect size. The Pillai trace became 0.5. Power analysis was conducted again to determine the actual power of the study.
-
•
Power analysis (using G*Power version 3.0.10) revealed that 29 participants were sufficient to produce a power level of 95% with a detected effect size of 1.00.
This analysis was conducted using SPSS for Windows, version 18 (SPSS, Inc, Chicago, Illinois). Before final analysis, data were screened for normality assumption, homogeneity of variance, and presence of extreme scores.
Descriptive analysis using histograms with the normal distribution curve showed that the data were normally distributed and did not violate the parametric assumption for any of the measured dependent variables. Additionally, testing for homogeneity of covariance revealed that there was no significant difference (P ≥ .05). Normality of the data was assessed using the Shapiro-Wilk test, which reflected that the data were normally distributed for 2MWD, 10mWT, EDSS, A/C, LR, and MMSE. Box and whisker plots were constructed of each of the tested variables after removal of the outliers. All these findings allowed us to conduct parametric analysis. Accordingly, a 2 × 2 mixed-design multivariate analysis of variance was used to compare the tested variables of interest in different tested groups (before and after rehabilitation). Changes associated with rehabilitation were calculated as differential before-and-after (delta) scores. The magnitude of change resulting from the intervention was calculated using Cohen's d. All statistical analyses had 2-tailed α levels of <0.05 for defining significance.
Results
Disease-modifying medications used by the patients are listed in Table 1. The demographics and clinical characteristics of both groups are listed in Table 2. Both groups were matched regarding age, sex, duration of illness, and relapse rate. Moreover, before any intervention, participants in both groups had comparable scores for EDSS (P = .68), MMSE (P = .878), LR (P = .05), A/C (P = .5), and walking tests (P = .71 for 2MWD and P = .343 for 10mWT). Mean values of these parameters are presented in Table 3. After completion of the rehabilitation program, the control group showed a statistically significant improvement in MMSE (P = .01), A/C (P = .004), and 10mWT (P = .000), but not in the other studied parameters. Regarding the study group, the postrehabilitation scores on all studied parameters were significantly better (all P values = .000).
Table 1.
Disease-Modifying Agents Used by the Participants
| Medication | Number (%) of Participants |
|---|---|
| Interferon beta-1a | 14 (48.3) |
| Azathioprine | 6 (20.7) |
| Cyclophosphamide | 3 (10.3) |
| Mitoxantrone | 1 (3.4) |
| Methotrexate injection | 1 (3.4) |
| Monthly methylprednisolone | 1 (3.4) |
| None | 3 (10.3) |
Table 2.
Demographics and Clinical Characteristics of Participants in the Study and Control Groups
| Variable | Study Group (n = 15) | Control Group (n = 14) | P |
|---|---|---|---|
| Age (y) | 30.80 (7.27) | 35.50 (8.95) | .13 |
| Duration of illness (y) | 7.43 (5.62) | 7.92 (2.61) | .766 |
| Relapses/y | 1.27 (0.75) | 1.02 (0.72) | .36 |
| Women/men, number (%) | 10 (66.6)/5 (33.4) | 11 (73.3)/4 (26.7) | .68 |
Data are presented as mean (SD), except where noted otherwise.
Table 3.
Scores of the Study and Control Groups Before and After Rehabilitation
| Group | EDSS | MMSE | A/C | LR | 2MWD (m) | 10mWT (s) | |
|---|---|---|---|---|---|---|---|
| Prerehabilitation, mean (SD) | Study group | 5.03 (0.51) | 26.2 (2.0) | 8.8 (1.26) | 3.66 (2.49) | 100.26 (42.35) | 12.8 (2.8) |
| Control group | 4.92 (0.82) | 26.07 (2.46) | 8.42 (1.65) | 2.21 (0.89) | 106.42 (46.07) | 14.35 (5.54) | |
| Postrehabilitation, mean (SD) | Study group | 4.33 (0.69) | 27.13 (2.13) | 10.53 (1.24) | 4.86 (2.66) | 159.2 (61.34) | 10.06 (2.15) |
| Control group | 4.78 (0.97) | 26.57 (2.24) | 9.07 (1.81) | 2.21 (0.89) | 116.78 (46.08) | 12.5 (4.27) | |
| Mean difference (post-pre) | Study group | 0.7 | −0.93 | −1.733 | −1.2 | −58.933 | 2.733 |
| Control group | 0.143 | −0.5 | −0.643 | 0.000 | −10.357 | 1.857 | |
| Effect sizea | Cohen's d | 0.009 | 0.007 | 0.096 | 0.231 | 0.042 | 0.069 |
| P | .896 | .495 | .017* | .001* | .046* | .061 | |
| 95% confidence interval | Upper bound | −1.178 | −1.106 | 0.282 | 1.113 | 0.831 | −4.985 |
| Lower bound | 1.340 | 2.230 | 2.642 | 4.192 | 83.997 | 0.119 |
A/C, attention/concentration; EDSS, Expanded Disability Status Scale; LR, logical reasoning; MMSE, Mini-Mental State Examination; 2MWD, 2-minute walking distance; 10mWT, 10-meter walking time.
Effect size, P values, and 95% confidence intervals refer to between-groups comparisons regarding (post-pre) delta values
significant P value.
Moreover, the differential before–after (delta) scores were compared between the study and control groups. Although the study group performed better than the control group on all tests, significant differences were observed only in LR, A/C, and 2MWD scores (Table 3).
Discussion
Over time, accumulation of damage to the central nervous system (due to inflammatory and degenerative processes) leads to impaired ambulation and cognition in individuals with MS, underscoring the importance of managing these burdensome co-occurring interacting consequences.19 Our participants were evaluated using EDSS, MMSE, A/C and LR capabilities, and walking tests (2MWD and 10mWT). As the last 4-minute period of the 6-meter walking distance test seems to be redundant, the 2MWD is considered as an alternative.20 The control group demonstrated improvement in not only ambulatory ability (10mWT) but also MMSE and A/C scores. Consistent with our findings, a meta-analysis21 including data from 13 randomized, controlled trials with 655 participants with MS who engaged in exercise training compared with nonexercise control conditions reported 16.5% improvement in 10mWT. Furthermore, rehabilitation programs that often combine different modalities of exercise22 have shown evidence of improvement in walking performance for individuals with MS. On the other hand, reports of improvement in cognitive outcomes after exercise training are rare and inconsistent.23 However, an earlier trial reports improvement in some domains of cognition after aerobic exercise training in individuals with MS.24
In the current study, with dual-task rehabilitation we demonstrated co-occurring significant improvements in EDSS, cognitive measures (MMSE, A/C, and LR), and walking performance (10mWT and 2MWD). Moreover, the magnitude of improvements in 2MWD, A/C, and LR scores in the study group significantly exceeded that in the control group. This is consistent with previous results on other neurologic diseases.25,26 The efficacy of this rehabilitation strategy in MS might differentially depend on the disability status27 or the domain of cognitive impairment.28 Of note, a desirable effect on impaired cognition in individuals with MS has been achieved with both selective29 and broader30 approaches to cognitive rehabilitation. In contrast, other authors using computerized approaches to rehabilitate attention and working memory simultaneously have failed to show a clear benefit.31
Proposed Mechanisms
The improvement in 10mWT in the control group after physical training was expected according to the task automatization hypothesis, which states that “practicing only one task at a time allows participants to automatize the performance of individual tasks with reduction in the processing demand required.”32 This achievement and the associated improvement in mental function could be explained by means of peripheral mechanisms—namely enhanced aerobic capacity (cardiorespiratory function), muscle strength, and postural control33,34—besides central neural mechanisms, namely adaptive plasticity. Aerobic exercise training and cardiorespiratory fitness have been shown to improve the structure and connectivity of the hippocampus in individuals with MS and memory impairment24 and to correlate with the volumes of the thalamus, hippocampus, and basal ganglia nuclei.35
Classically, researchers have separately evaluated physical training as an approach for improving walking and cognitive training as an approach for improving cognitive function. In the current study, coupled physical and cognitive training was associated with broad and amplified improvement in mental and ambulatory functions. There is an increasing body of evidence suggesting cross-modality transfer effects of exercise and cognitive rehabilitation on cognition and motor outcomes.9 Deficits in attention and executive function are independently associated with the risk of postural instability, falls, and impairment in daily activities.6 A positron emission tomography study36 has demonstrated an association between increased complexity of gait (ie, obstacle avoidance) and increased activity in the hippocampus, which is functionally connected through the entorhinal cortex and nigrostriatal system to the prefrontal cortex, the region involved in executive function. Moreover, the observation that performing a cognitive task concurrently while walking is associated with a reduction in walking performance supports the notion that gait speed-control areas may be interlinked with the networks of executive functions. Therefore, cognitive–motor interference while walking may occur when the concurrent tasks compete for these shared neural networks (bottleneck theory).37 Alternatively, each kind of task draws from limited attention resources; hence, if the resources required by the 2 tasks exceed the limited brain capacity, a cognitive–motor interference will arise (capacity-sharing theory).38 Therefore, compensatory adaptations in structures, function, and connectivity in the central nervous system are proposed mechanisms for improvements of walking and cognitive outcomes in MS, and this adaptive plasticity has proven to be enhanced in brain networks specifically subserving the trained function (task-oriented rehabilitation).39 The dual-task training in the current study targeted cognitive domains sharing networks involved in walking. Therefore, the improvement in walking speed (2MWD) in the study group was accompanied by parallel improvement in attention and logical reasoning and exceeded that achieved by the control group. Indeed, there are bidirectional paths such that walking and cognitive consequences of MS could influence one another and hence the quality of an individual's life.
Limitation
Despite the encouraging findings, the current study suffers from some drawbacks, mainly the lack of extended reevaluation to check whether the benefit of training was retained for longer periods after the end of the rehabilitation program. Applicability of the current study finding is limited to the selected sample and the applied rehabilitation program.
Conclusion
The coupled exercise and cognitive (dual-task) training showed concurrent improvement in cognitive and walking abilities in individuals with RRMS which exceeded that achieved by exercise training alone.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): A.E., A.M.E.
Design (planned the methods to generate the results): A.E., A.M.E.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): A.M.E., A.S.A.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): A.A.M.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): A.M.E., A.A.M.
Literature search (performed the literature search): A.A.M.
Writing (responsible for writing a substantive part of the manuscript): A.S.A., A.E.E.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): A.M.E., A.S.A.
Practical Applications.
-
•
This study showed that dual task training improved physical disability and cognitive performance of MS patients better than physical training only
-
•
Dual task training was beneficial in improving quality of life of MS patients.
-
•
Rehabilitation protocol used in this study decreased economic burden that results from physical and cognitive disabilities in MS patients
Alt-text: Unlabelled box
References
- 1.Goldberg L, Edwards NC, Fincher C, Doan QV, Al-Sabbagh A, Meletiche DM. Comparing the cost-effectiveness of disease-modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosis. J Manag Care Spec Pharm. 2009;15(7):543–555. doi: 10.18553/jmcp.2009.15.7.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiner HL. A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J Neurol. 2008;255(1):3–11. doi: 10.1007/s00415-008-1002-8. [DOI] [PubMed] [Google Scholar]
- 3.Loma I, Heyman R. Multiple sclerosis: pathogenesis and treatment. Curr Neuropharmacol. 2011;9(3):409–416. doi: 10.2174/157015911796557911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Tahan AM, Alsharoqi I, Bohlega SA. Characteristics of multiple sclerosis in the Middle East with special reference to the applicability of international guidelines to the region. Int J Neurosci. 2014;124(9):635–641. doi: 10.3109/00207454.2013.865620. [DOI] [PubMed] [Google Scholar]
- 5.Hemmer B, Nessler S, Zhou D, Kieseier B, Hartung H-P. Immunopathogenesis and immunotherapy of multiple sclerosis. Nat Clin Pract Neurol. 2006;2(4):201–211. doi: 10.1038/ncpneuro0154. [DOI] [PubMed] [Google Scholar]
- 6.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23(3):329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruet A, Deloire M, Hamel D, Ouallet J-C, Petry K, Brochet B. Cognitive impairment, health-related quality of life and vocational status at early stages of multiple sclerosis: a 7-year longitudinal study. J Neurol. 2013;260(3):776–784. doi: 10.1007/s00415-012-6705-1. [DOI] [PubMed] [Google Scholar]
- 8.Leone C, Patti F, Feys P. Measuring the cost of cognitive-motor dual tasking during walking in multiple sclerosis. Mult Scler J. 2015;21(2):123–131. doi: 10.1177/1352458514547408. [DOI] [PubMed] [Google Scholar]
- 9.Motl RW, Sandroff BM, DeLuca J. Exercise training and cognitive rehabilitation: a symbiotic approach for rehabilitating walking and cognitive functions in multiple sclerosis. Neurorehabil Neural Repair. 2016;30(6):499–511. doi: 10.1177/1545968315606993. [DOI] [PubMed] [Google Scholar]
- 10.Law LLF, Barnett F, Yau MK, Gray MA. Effects of combined cognitive and exercise interventions on cognition in older adults with and without cognitive impairment: a systematic review. Ageing Res Rev. 2014;15:61–75. doi: 10.1016/j.arr.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Polman CH, Reingold SC, Banwell B. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-MentalState”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Fernández E, Bringas ML, Salazar S, Rodríguez D, García ME, Torres M. Clinical impact of RehaCom software for cognitive rehabilitation of patients with acquired brain injury. MEDICC Rev. 2012;14(4):32–35. doi: 10.37757/MR2012V14.N4.8. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 16.Andersson G, Hagman J, Talianzadeh R, Svedberg A, Larsen HC. Effect of cognitive load on postural control. Brain Res Bull. 2002;58(1):135–139. doi: 10.1016/s0361-9230(02)00770-0. [DOI] [PubMed] [Google Scholar]
- 17.Silsupadol P, Siu K-C, Shumway-Cook A, Woollacott MH. Training of balance under single- and dual-task conditions in older adults with balance impairment. Phys Ther. 2006;86(2):269–281. [PubMed] [Google Scholar]
- 18.Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J. Cognitive motor interference while walking: A systemic review and meta-analysis. Neurosci Biobehav Rev. 2011;35(3):715–728. doi: 10.1016/j.neubiorev.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Benedict RHB, Holtzer R, Motl RW, Foley FW, Kaur S, Hojnacki D, Weinstock-Guttman B. Upper and lower extremity motor function and cognitive impairment in multiple sclerosis. J Int Neuropsychol Soc. 2011;17(4):643–653. doi: 10.1017/S1355617711000403. [DOI] [PubMed] [Google Scholar]
- 20.Gijbels D, Eijnde BO, Feys P. Comparison of the 2- and 6-minute walk test in multiple sclerosis. Mult Scler J. 2011;17(10):1269–1272. doi: 10.1177/1352458511408475. [DOI] [PubMed] [Google Scholar]
- 21.Pearson M, Dieberg G, Smart N. Exercise as a therapy for improvement of walking ability in adults with multiple sclerosis: a meta-analysis. Arch Phys Med Rehabil. 2015;9(7):1339–1348.e7. doi: 10.1016/j.apmr.2015.02.011. 6. [DOI] [PubMed] [Google Scholar]
- 22.Sangelaji B, Nabavi SM, Estebsari F, Banshi MR, Rashidian H, Jamshidi E, Dastoorpour M. Effect of combination exercise therapy on walking distance, postural balance, fatigue and quality of life in multiple sclerosis patients: a clinical trial study. Iran Red Crescent Med J. 2014;16(6):e17173. doi: 10.5812/ircmj.17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonnell MN, Smith AE, Mackintosh SF. Aerobic exercise to improve cognitive function in adults with neurological disorders: a systematic review. Arch Phys Med Rehabil. 2011;92(7):1044–1052. doi: 10.1016/j.apmr.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Leavitt VM, Cirnigliaro C, Cohen A. Aerobic exercise increases hippocampal volume and improves memory in multiple sclerosis: preliminary findings. Neurocase. 2014;20(6):695–697. doi: 10.1080/13554794.2013.841951. [DOI] [PubMed] [Google Scholar]
- 25.Yogev-Seligmann G, Giladi N, Brozgol M, Hausdorff JM. A training program to improve gait while dual tasking in patients with Parkinson's disease: a pilot study. Arch Phys Med Rehabil. 2012;93(1):176–181. doi: 10.1016/j.apmr.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Kim GY, Han MR, Lee HG. Effect of dual-task rehabilitative training on cognitive and motor function of stroke patients. J Phys Ther Sci. 2014;26(1):1–6. doi: 10.1589/jpts.26.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandroff BM, Klaren RE, Pilutti LA, Dlugonski D, Benedict RHB, Motl RW. Randomized controlled trial of physical activity, cognition, and walking in multiple sclerosis. J Neurol. 2014;261(2):363–372. doi: 10.1007/s00415-013-7204-8. [DOI] [PubMed] [Google Scholar]
- 28.Chiaravalloti ND, DeLuca J. The influence of cognitive dysfunction on benefit from learning and memory rehabilitation in MS: a sub-analysis of the MEMREHAB trial. Mult Scler J. 2015;21(12):1575–1582. doi: 10.1177/1352458514567726. [DOI] [PubMed] [Google Scholar]
- 29.Chiaravalloti ND, Wylie G, Leavitt V, DeLuca J. Increased cerebral activation after behavioral treatment for memory deficits in MS. J Neurol. 2012;259(7):1337–1346. doi: 10.1007/s00415-011-6353-x. [DOI] [PubMed] [Google Scholar]
- 30.Mattioli F, Stampatori C, Zanotti D, Parrinello G, Capra R. Efficacy and specificity of intensive cognitive rehabilitation of attention and executive functions in multiple sclerosis. J Neurol Sci. 2010;288(1-2):101–105. doi: 10.1016/j.jns.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Mäntynen A, Rosti-Otajärvi E, Koivisto K, Lilja A, Huhtala H, Hämäläinen P. Neuropsychological rehabilitation does not improve cognitive performance but reduces perceived cognitive deficits in patients with multiple sclerosis: a randomized, controlled, multi-centre trial. Mult Scler J. 2014;20(1):99–107. doi: 10.1177/1352458513494487. [DOI] [PubMed] [Google Scholar]
- 32.Ruthruff E, Van Selst M, Johnston JC, Remington R. How does practice reduce dual-task interference: integration, automatization, or just stage-shortening? Psychol Res. 2006;70(2):125–142. doi: 10.1007/s00426-004-0192-7. [DOI] [PubMed] [Google Scholar]
- 33.Motl RW. Physical activity and irreversible disability in multiple sclerosis. Exerc Sport Sci Rev. 2010;38(4):186–191. doi: 10.1097/JES.0b013e3181f44fab. [DOI] [PubMed] [Google Scholar]
- 34.Sandroff BM, Klaren RE, Motl RW. Relationships among physical inactivity, deconditioning, and walking impairment in persons with multiple sclerosis. J Neurol Phys Ther. 2015;39(2):103–110. doi: 10.1097/NPT.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 35.Motl RW, Pilutti LA, Hubbard EA, Wetter NC, Sosnoff JJ, Sutton BP. Cardiorespiratory fitness and its association with thalamic, hippocampal, and basal ganglia volumes in multiple sclerosis. Neuroimage Clin. 2015;7:661–666. doi: 10.1016/j.nicl.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp. 2003;19(1):47–62. doi: 10.1002/hbm.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruthruff E, Pashler HE, Klaassen A. Processing bottlenecks in dual-task performance: structural limitation or strategic postponement. Psychon Bull Rev. 2001;8(1):73–80. doi: 10.3758/bf03196141. [DOI] [PubMed] [Google Scholar]
- 38.Tombu M, Jolicœur P. A central capacity-sharing model of dual-task performance. J Exp Psychol Hum Percept Perform. 2003;29(1):3–18. doi: 10.1037//0096-1523.29.1.3. [DOI] [PubMed] [Google Scholar]
- 39.Prosperini L, Fanelli F, Petsas N. Multiple sclerosis:changes in microarchitecture of white matter tracts after training with a video game balance board. Radiology. 2014;273(2):529–538. doi: 10.1148/radiol.14140168. [DOI] [PubMed] [Google Scholar]