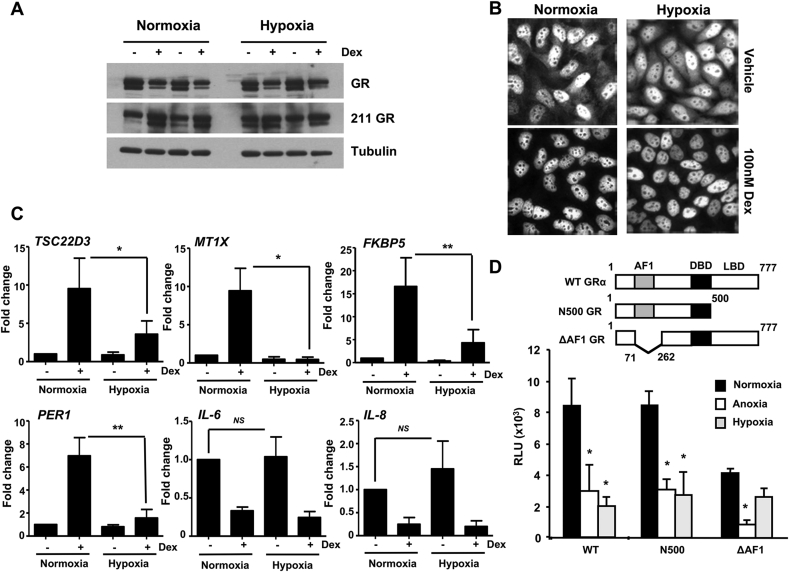
Fig. 8.
Hypoxia regulates GR function through transactivation domain. (A) HeLa cells were cultured in either normoxia or hypoxia overnight, treated with 100 nM dexamethasone (Dex) for 1 h then lysed and immunoblotted for GR and Ser211 phosphorylated GR. Tubulin was used as a loading control. Samples from two independent experiments are shown. (B) Cells cultured in normoxia or hypoxia overnight were treated with 100 nM dexamethasone (Dex) then fixed, and immunolabelled for GR (white) expression. (C) Cells were cultured under normoxic or hypoxic conditions overnight, then treated with dexamethasone for 4 h prior to lysis. qRT-PCR was used to quantify GR transactivation of TSC22D3, MT1X, FKBP5, and PER1 and transrepression of IL-6 and IL-8. Graphs show mean ± S.D. of experiments performed in triplicate and repeated three times. **p < 0.001, *p < 0.05, compared to normoxia. NS: not significant. (D) HEK293T cells expressing 2 μg TAT3-luc together with full length wild type receptor, GR ΔAF1 (lacking N-terminus), or GR N500 (lacking C-terminus) were cultured under normoxia with vehicle or 100 μM deferoxamine (hypoxia), or cultured in anoxia overnight. Cells were incubated with dexamethasone for 16 h before luciferase assay. Graph depicts mean ± S.D. and is representative of at least three independent experiments. *p < 0.05 compared to normoxia.