Figure 1 |. Pf NfnI structure and orientation of cofactors.
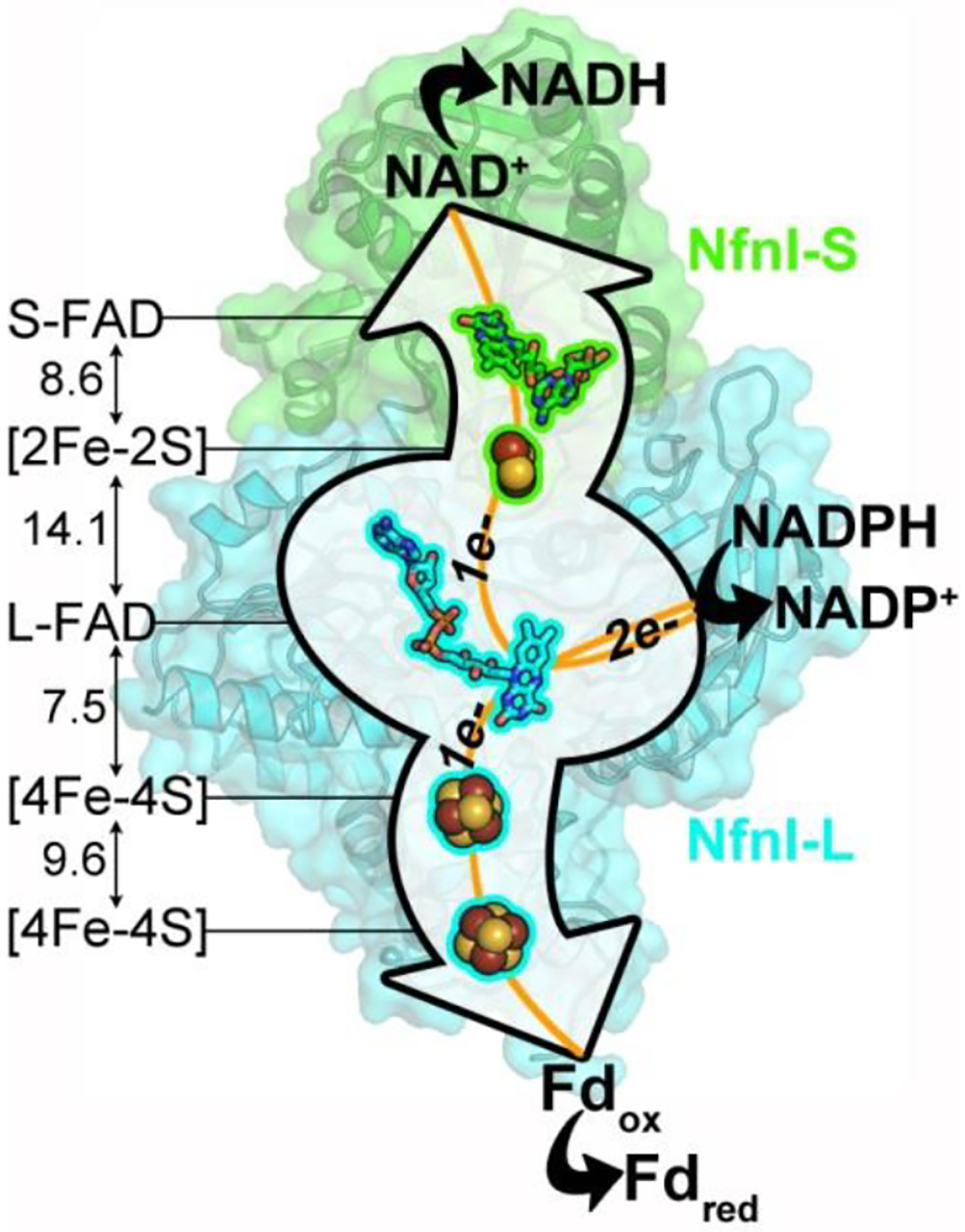
Pf NfnI is a heterodimer of 31 kDa NfnI-S and 53 kDa NfnI-L. NfnI-S contains S-FAD and a [2Fe-2S] cluster with an unusual Asp ligand. NfnI-L contains one FAD (L-FAD), which is the site of electron bifurcation, and two [4Fe-4S] clusters. The L-FAD proximal [4Fe-4S] cluster coordination includes an unusual Glu ligand. S-FAD and L-FAD bind NADH and NADPH, respectively. Ferredoxin (Fd) is proposed to bind near the distal [4Fe-4S] cluster of NfnI-L. Edge-to-edge distances between cofactors are given in Ångström (Å). Pf NfnI shows sequence and structural similarity to the flavin-based electron bifurcating enzyme from Thermotoga maritima (NfnAB, PDB 4YRY). Fdox, oxidized Fd; Fdred, reduced Fd.
