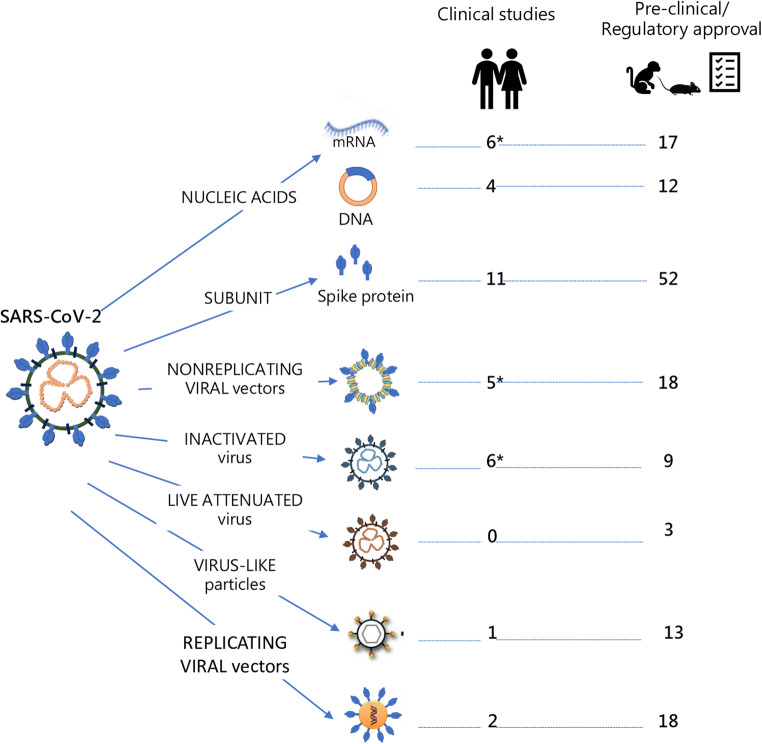
Fig. 4.
SARS-CoV-2 vaccine candidates based on different vaccine platforms. Schematic representation of the different vaccine platforms used for developing SARS-CoV-2 vaccines. These include nucleic acid (both mRNA and DNA); subunit S protein with different adjuvants; non-replicating viral vectors (such as Adenovirus); inactivated SARS-CoV-2 virus alone or combined with adjuvants; live SARS-CoV-2 attenuated virus; virus-like particles and replicating viral vectors (such as Measles virus, Influenza virus, Vesicular stomatitis virus, and others). About 140 vaccine candidates are currently involved in pre-clinical studies, while 35 vaccine candidates are worldwide tested in clinical studies, and some of them (indicated with *) have already reached the phase III. For each platform, the number of ongoing clinical or pre-clinical studies is reported. Data are referred to the WHO report, updated to 17 September 2020