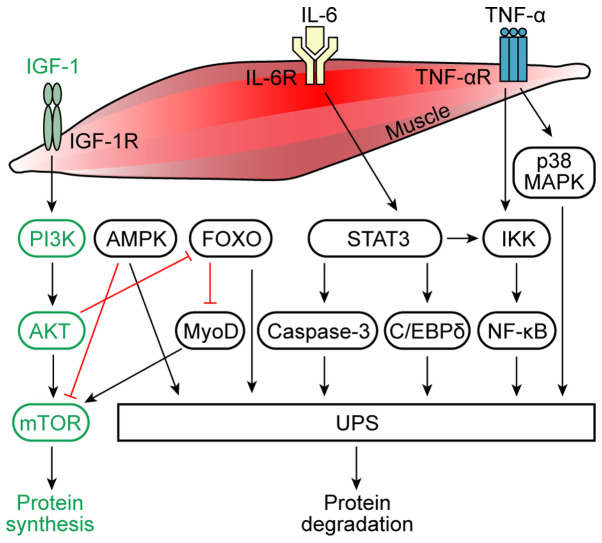
Figure 1.
Molecules and signaling pathways involved in muscle protein synthesis and degradation. Under physiological conditions, IGF-1 activates AKT through a PI3K-dependent process, leading to the activation of mTOR and thus resulting in the increased proliferation of muscle cells and increased protein synthesis in muscle cells. IL-6 and TNF-α are considered to be the main mediators of the inflammation in muscle atrophy caused by cancer cachexia. The binding of IL-6 to its receptor induces AMPK and STAT3 expression. STAT3 induces the activation of the IKK/NF-κB pathway and caspase-3 and C/EBPδ expression, which activates the UPS, causing muscle protein degradation. AMPK is a downstream target of IL-6 signaling, which inhibits the mTOR cascade, and activates the UPS. Physiologically, AKT inhibits FOXO, which promotes protein synthesis. In cancer cachexia, FOXO inhibits MyoD and activates the UPS, thereby promoting protein degradation. Moreover, the activation of NF-κB due to the degradation of the IκB inhibitor by IKK is TNF-α-dependent. The NF-κB pathway can further activate the UPS to cause muscle protein degradation. TNF-α can also induce the p38 MAPK pathway, which activates the UPS. IGF-1, insulin-like growth factor 1; AMPK, AMP-activated protein kinase; IKK, IκB kinase; C/EBPδ, CCAAT/enhancer-binding protein δ; UPS, ubiquitin-proteasome system; MyoD, myoblast determination protein 1; R, receptor.