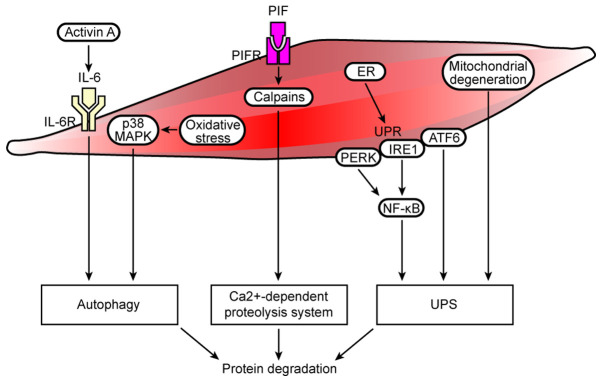
Figure 2.
Cell autophagy/lysosomal and Ca2+-dependent protein degradation pathways, ER stress and mitochondrial dysfunction are involved in muscle protein degradation in cancer cachexia. In cancer cachexia, increased activin A expression activates the IL-6 signaling pathway, and oxidative stress induces activation of the p38 MAPK signaling pathway, resulting in autophagy and protein degradation. The overexpression of Ca2+-dependent proteases (calpains) activates the Ca2+-dependent proteolysis system, resulting in increased protein degradation. In addition, the ER manages such stress by initiating the UPR, which is controlled by three transmembrane proteins, namely, PERK, IRE1 and ATF6. Optimal activation of NF-κB during ER stress requires inputs from both IRE1 and PERK activities, and ATF6 may interact with protein degradation pathways, such as the UPS. Moreover, local mitochondrial degeneration in the muscle activates the UPS, which results in protein degradation. R, receptor; PERK, protein kinase-like ER eukaryotic translation initiation factor 2α kinase; ER, endoplasmic reticulum; UPR, unfolded protein response; IRE1, inositol-requiring protein; ATF6, activating transcription factor 6; UPS, ubiquitin-proteasome system.