Abstract
Mechanical ventilation (MV) can contribute to ventilator-induced lung injury (VILI); dexmedetomidine (Dex) treatment attenuates MV-related pulmonary inflammation, but the mechanisms remain unclear. Therefore, the present study aimed to explore the protective effect and the possible molecular mechanisms of Dex in a VILI rodent model. Adult male Sprague-Dawley rats were randomly assigned to one of seven groups (n=24 rats/group). Rats were euthanized after 4 h of continuous MV, and pathological changes, lung wet/dry (W/D) weight ratio, the levels of inflammatory cytokines (IL-1β, TNF-α and IL-6) in the bronchoalveolar lavage fluid (BALF), and the expression levels of Bcl-2 homologous antagonist/killer (Bak), Bcl-2, pro-caspase-3, cleaved caspase-3 and the phosphorylation of ERK1/2 in the lung tissues were measured. Propidium iodide uptake and TUNEL staining were used to detect epithelial cell death. The Dex pretreatment group exhibited fewer pathological changes, lower W/D ratios and lower expression levels of inflammatory cytokines in BALF compared with the VILI group. Dex significantly attenuated the ratio of Bak/Bcl-2, cleaved caspase-3 expression levels and epithelial cell death, and increased the expression of phosphorylated ERK1/2. The protective effects of Dex could be partially reversed by PD98059, which is a mitogen-activated protein kinase (upstream of ERK1/2) inhibitor. Overall, dexmedetomidine was found to reduce the inflammatory response and epithelial cell death caused by VILI, via the activation of the ERK1/2 signaling pathway.
Keywords: dexmedetomidine, ventilator-induced lung injury, ERK1/2
Introduction
As an effective mode of treatment for acute respiratory distress syndrome (ARDS), mechanical ventilation (MV) is widely performed in patients under general anesthesia (1). However, certain MV strategies can result in ventilator-associated complications, such as ventilator-induced lung injury (VILI) (2–4). In addition to mechanical damage, there is growing evidence that upregulation of inflammatory molecules, chemokines, leukocyte sequestration and excessive inflammatory responses has a vital role in the generation and progression of VILI (5–7). However, the definite mechanism through which this pro-inflammatory response is initiated and sustained, and how to relieve it, remain unclear.
Recently, it was reported that dexmedetomidine (Dex) exhibits analgesic and sedative properties for systemic anesthesia by intravenous (i.v.) infusion through the activation of α2 adrenergic receptors (8). In patients subjected to MV, Dex has been widely used in intensive care units. Ample evidence has been reported on the protective effects of MV on organs (9). It has been demonstrated that Dex could ameliorate VILI-induced pulmonary inflammation in animal models (10,11); Chen et al (12) observed that dex reduces VILI induced inflammation by inhibiting TLR4 and NF-κB signaling through α2-adrenoreceptors. However, the exact mechanism has yet to be established. It was hypothesized that the protective signaling pathways in the lung cells are initiated through Dex for the prevention of cellular apoptosis or necrosis when subjected to MV.
ERK1/2 is a member of the MAPK family, and serves a key role in the regulation of cell death and survival (13–15). The inhibition of ERK1/2 signaling induces the activation of apoptosis-associated proteins, such as BH3-interacting domain death agonist, Bcl-2-like protein 11 and p53 upregulated modulator of apoptosis. In addition, the MEK1/2-ERK1/2 signaling pathway may be involved in modulating the inflammatory response to lung injury and infection (16). However, little attention has been dedicated to the effects of Dex administration with respect to ERK1/2 and the expression of caspase-3, Bcl-2 and Bcl-2 homologous antagonist/killer (Bak) in VILI. Therefore, the present study aimed to provide evidence that supports Dex as a treatment against VILI in a rat model of high-tidal volume MV (HVT-MV) and to investigate the roles of phosphorylated (p)-ERK1/2.
Materials and methods
Animals
A total of 168 adult male Sprague-Dawley rats (6–8 weeks old, 200–250 g) were purchased from Liaoning Changsheng Biotechnology and housed one per cage at a stable temperature of 25±1°C with alternating 12-h light/dark cycle. Rats were allowed access to food and water ad libitum until the start of the experiments. All study procedures and animal handling were approved by the Animal Review Board of Cangzhou Central Hospital (Cangzhou, China) and were in accordance with the National Institutes of Health guidelines.
Group assignment and experimental protocol
After successful sevoflurane anesthesia (5% for induction; 1.5–2.5% for maintenance), an indwelling needle (22G) was placed into the catheterized caudal (tail) vein. The left femoral artery was cannulated with a heparinized tube to monitor arterial blood pressure. The level of sevoflurane was adjusted to maintain stable hemodynamics. According to computer-based randomization, the rats were assigned to one of the following seven groups: i) Sham; ii) VILI; iii) VILI+low dose (L)-Dex; iv) VILI+high dose (H)-Dex; v) VILI+L-Dex+PD98059 (PD; Calbiochem; Merck KGaA); vi) VILI+H-Dex+PD; and vii) PD only group. In the Sham group, rats were anesthetized with sevoflurane and kept on spontaneous breathing without MV for 4 h, and IV catheterization was performed. In the VILI group, the endotracheal intubation tube was connected to a ventilator (model 683 Ventilator; Harvard Apparatus) for 4 h. MV with a high tidal volume of 20 ml/kg, respiratory frequency of 50 bpm, and compressed air was used. Each rat could achieve a peak airway pressure of ~40 cm H2O, as measured by an Exactus II pressure gauge (Omron Healthcare Inc.). VILI+L-Dex group rats received the same MV strategy as those in the VILI group and received i.v. administration of a loading dose of Dex (Jiangsu Hengrui Medicine Co., Ltd.) of 1 µg/kg over 15 min before MV, followed by a maintenance dose of 1 µg/kg/h (i.v.) during MV. Similarly, rats in the VILI+H-Dex group received the same MV strategy and received i.v. administration of 10 µg/kg for loading dose and 10 µg/kg/h for maintenance dose (17,18). Rats in the VILI+L-Dex+PD group and the VILI+H-Dex+PD received subcutaneous injections of 1 mg/kg PD, an ERK1/2 inhibitor (19,20), 30 min prior to MV and Dex infusion. The dose of 1 mg/kg was based on a preliminary experiment and a previous study (21). Subsequently, the ventilation strategy of the rats and the infusion of Dex were consistent with the VILI+L-Dex group and the VILI+H-Dex groups. Finally, rats in the PD group received 1 mg/kg PD subcutaneously.
At the end of each experiment, the lungs and bronchoalveolar lavage fluid (BALF) were collected under anesthesia with sevoflurane. The left lung lobe was used for determining the lung wet/dry (W/D) weight ratio after removing the blood and water from the lung surface.
Histopathological analysis
After fixing in 4% paraformaldehyde at room temperature overnight, the lung tissues were dehydrated and embedded in paraffin. The tissue sections (thickness, 4 µm) were stained with hematoxylin and eosin (H&E). The images were captured under a light microscope and then assessed by a pathologist who was blinded to the groups. Each sample was evaluated according to the following three criteria: i) Alveolar edema; 0, no edema in the interstitium; 1, slight edema in the interstitium wit hyaline membrane formation; 2, interstitial edema is evident; 3, interstitial edema is further aggravated and hyaline membrane was formed. ii) Diffuse alveolar hemorrhage and congestion; 0, none; 1, prominent congestion with extravasated blood; 2, <25% extravasated blood; and 3, >25% extravasated blood. iii) Intra-alveolar infiltration of inflammatory cells; 0, none; 1, <10% aggregates; 2, 10–30% aggregates; and 3, >30% aggregates. The indexes of the final score are accumulated.
ELISA
Interleukin-6 assay kit (cat. no. H007), interleukin-1β assay kit (cat. no. H002) and tumor necrosis factor-α (TNF-α) assay kit (cat. no. H052) were purchased from Nanjing Jiancheng Bioengineering Institute and were used to measure IL-6, IL-1β and TNF-α levels, respectively, in the BALF according to the manufacturer's protocol.
Lung W/D weight ratio
Pulmonary edema development was assessed by the ratio of lung W/D weight. Briefly, the lung tissues were separated from the upper lobes of the left lung. After washing in saline, the tissues were weighed immediately and reweighed followed dehydration at 80°C for 24 h.
Immunofluorescence
To quantitatively measure alveolar epithelial cell death, the TUNEL/propidium iodide (PI)/Hoechst 33342 assay was used. After dewaxing with xylene and a serial ethanol gradient concentration (100% ethanol for 3 min, 95% ethanol for 2 min, 80% ethanol for 2 min, 75% ethanol for 2 min, H2O for 1 min), the tissue sections (4 µm) were incubated with 20 µg/ml proteinase K (cat. no. ST533; Beyotime Institute of Biotechnology) for 10 min at 37°C. Then, the slices were treated with TUNEL-mixture (cat. no. C1088; Beyotime Institute of Biotechnology) in the dark for 1 h at 37°C. After washing with PBS, 5 µg/ml of Antifade Mounting Medium with Hoechst 33342 and propidium iodide (PI; cat. no. P0137; Beyotime Institute of Biotechnology) were added for 2 min at room temperature to stain the cell nuclei and necrotic cell. Four nonoverlapping fields of view were evaluated in each tissue section. The percentage of necrosis and apoptosis was indicated by the ratio of PI-positive nuclei or TUNEL-positive nuclei to the total cell nuclei counterstaining by Hoechst 33342, respectively.
Western blot analysis
Western blotting was used to assess the expression in ERK1/2, caspase-3, Bcl-2 and Bak. Total protein was extracted from lung tissues and lysed in tissue lysis buffer (cat. no. P0013; Beyotime Institute of Biotechnology), and the concentration was measured using a bicinchoninic acid Protein Assay Kit (cat. no. P0012S; Beyotime Institute of Biotechnology). Samples containing 30 µg of total protein were separated on a 12% SDS-PAGE and transferred to a polyvinylidene fluoride membrane. The membranes were blocked with QuickBlock™ blocking buffer for western blot (Beyotime Institute of Biotechnology) at 25°C for 10 min, and then incubated with primary antibodies of rabbit monoclonal anti-ERK1/2 (1:1,000; cat. no. AF1051; Beyotime Institute of Biotechnology), anti-phospho-ERK1/2 (1:1,000; cat. no. AF1891; Beyotime Institute of Biotechnology) polyclonal anti-caspase-3 (1:1,000; cat. no. ab13847; Abcam), anti-Bak (1:500; cat. no. AB016; Beyotime Institute of Biotechnology) and anti-Bcl-2 (1:500; cat. no. K003505P; Beijing Solarbio Science & Technology Co., Ltd.) overnight at 4°C. After rinsing with TBS+Tween-20 (0.05%) buffer (Beyotime Institute of Biotechnology), the membranes were probed with HRP-conjugated goat anti-rabbit secondary antibodies (1:2,000; cat. no. A0208; Beyotime Institute of Biotechnology) for 1 h at 25°C. After incubation with an ECL plus kit (Beyotime Institute of Biotechnology) for 5 min, the blot was visualized and semi-quantified using Image Lab 5.1 software (Bio-Rad Laboratories, Inc.). GAPDH (1:1,000; cat. no. K106389P; Beijing Solarbio Science & Technology Co., Ltd.) was used as an internal reference.
Statistical analysis
All statistical analyses were performed using SPSS Statistics version 20.0 (IBM Corp). Data are expressed as the mean ± SD. When only two groups were compared, an unpaired t-test was used. Differences between more than two groups were assessed by one-way analyses of variance followed by a Bonferroni's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Dex attenuates histopathological damage in rat lungs
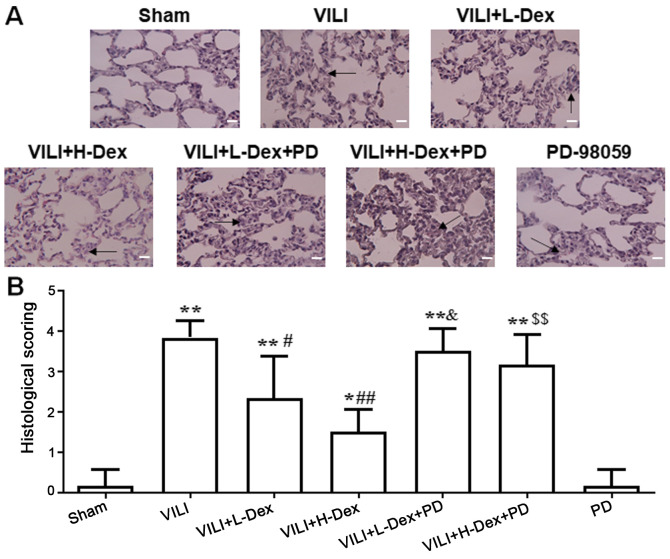
Lungs were stained with H&E to assess histopathology based on morphology and scoring. The structural changes and pulmonary edema in the VILI group were more serious compared with those in the Sham group (Fig. 1). Dex treatment alleviated the lung morphologic injury, especially in the VILI+H-Dex group compared with the VILI group. However, after inhibition with PD98059, the protective effect of Dex on morphology was weakened. PD98059 injection alone did not damage the lung tissue.
Figure 1.
Dex attenuates histopathological injury in rat lungs. (A) Hematoxylin and eosin staining of rat lung tissue (magnification, ×200; scale bar, 50 µm). The arrow indicates congestion and edema (B) The histopathological scores of degree of lung injury. Data are expressed as the mean ± SD (n=6). *P<0.05, **P<0.01 vs. Sham; #P<0.05, ##P<0.01 vs. VILI; &P<0.05 VILI+L-Dex+PD vs. VILI+L-Dex; $$P<0.01 VILI+H-Dex+PD vs. VILI+H-Dex. Dex, dexmedetomidine; H-Dex, high dose Dex at 10 µg/kg; L-Dex, low dose Dex at 1 µg/kg; PD, PD98059; VILI, ventilator-induced lung injury.
Dex was demonstrated to have little effect on hemodynamics
During the treatment process, the hemodynamics of rats were found to be relatively stable (Tables SI and SII). This indicated that hemodynamics have no effect on the pathology and inflammatory response.
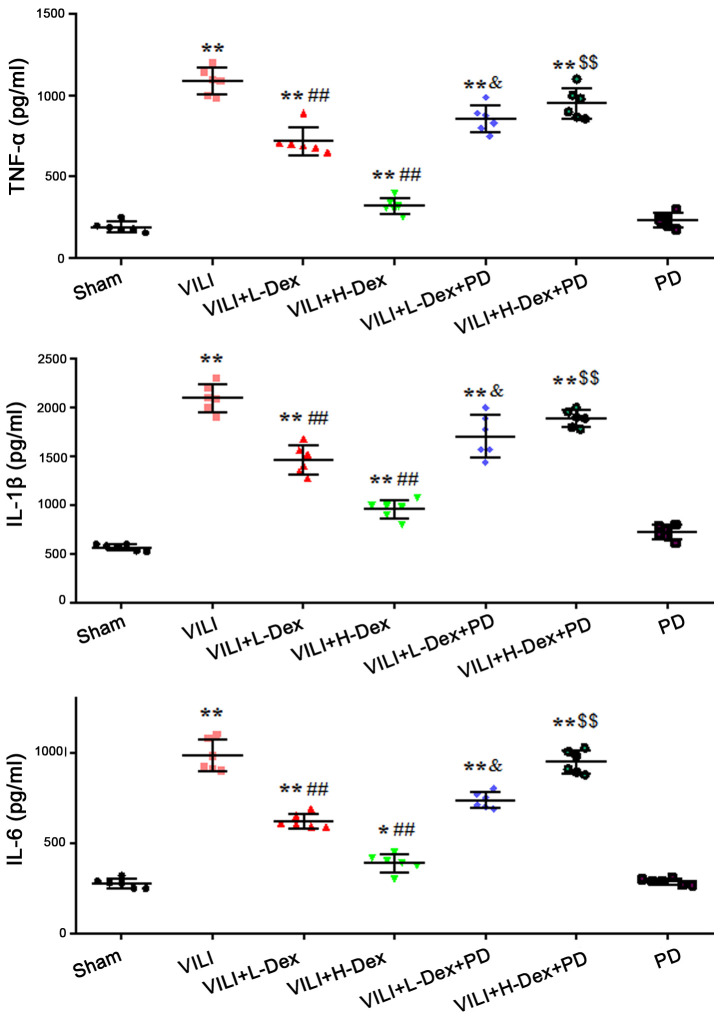
Dex reduces the level of inflammatory cytokines in BALF
The effects of Dex on inflammatory cytokine levels in BALF were measured using ELISAs. The results demonstrated that the production of TNF-α, IL-1β and IL-6 in the VILI group were significantly increased compared with levels in the Sham group (P<0.05; Fig. 2). L-Dex (P<0.05) and H-Dex (P<0.01) significantly downregulated the expression levels of inflammatory cytokines compared with the VILI group. PD98059 pretreatment significantly offset the effects of Dex in the VILI+L-Dex and VILI+H-Dex groups, compared with the VILI+L-Dex+PD and VILI+H-Dex+PD groups (both P<0.05; Fig. 2).
Figure 2.
Dex reduces the expression levels of inflammatory cytokines in BALF. The expression levels of TNF-α, IL-1β and IL-6 in the BALF of rats. The data are expressed as the mean ± SD (n=6). *P<0.05, **P<0.01 vs. Sham; ##P<0.01 vs. VILI group; &P<0.05 VILI+L-Dex+PD vs. VILI+L-Dex; $$P<0.01 VILI+H-Dex+PD vs. VILI+H-Dex. BALF, bronchoalveolar lavage fluid; Dex, dexmedetomidine; H-Dex, high dose Dex at 10 µg/kg; L-Dex, low dose Dex at 1 µg/kg; PD, PD98059; VILI, ventilator-induced lung injury.
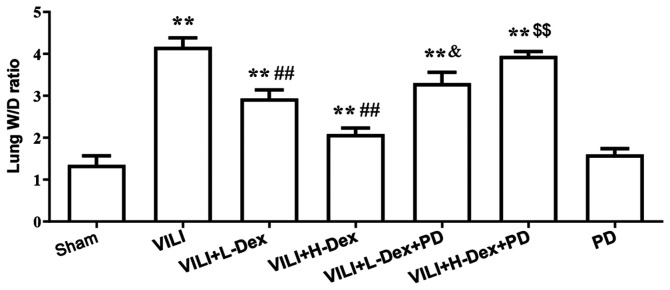
Dex reduces the lung W/D weight ratio
Pulmonary edema was evaluated by the W/D weight ratio of the lungs (Fig. 3). The W/D ratio in the VILI group was found to be significantly higher compared with that in the Sham group (P<0.05). In the VILI+L-Dex (P<0.05) and the VILI+H-Dex treatment groups (P<0.01), Dex significantly reduced the lung W/D ratios compared with the VILI group. PD98059 pretreatment was found to reverse the protective effects of Dex (P<0.05).
Figure 3.
Dex reduces the lung W/D ratio. The values of the W/D weight ratio in different groups are presented as mean ± SD (n=6). **P<0.01 vs. Sham; ##P<0.01 vs. VILI; &P<0.05 VILI+L-Dex+PD vs. VILI+L-Dex; $$P<0.01 VILI+H-Dex+PD vs VILI+H-Dex. Dex, dexmedetomidine; H-Dex, high dose Dex at 10 µg/kg; L-Dex, low dose Dex at 1 µg/kg; PD, PD98059; VILI, ventilator-induced lung injury; W/D, wet/dry.
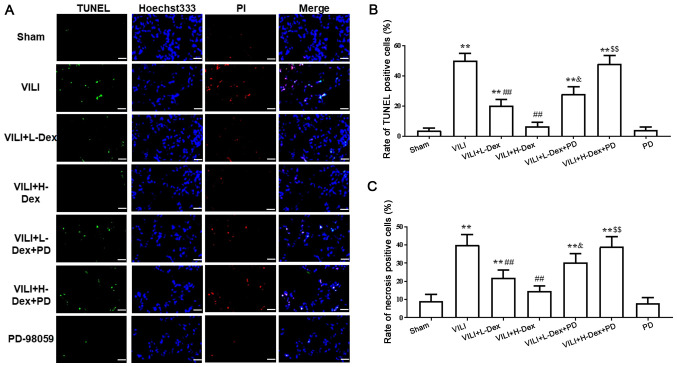
Dex reduces alveolar epithelial cell death
Immunofluorescence assays demonstrated that, compared with the Sham group, the necrosis and apoptosis of alveolar epithelial cells significantly increased in the VILI treated group (P<0.05; Fig. 4). However, a significant decrease was revealed in VILI model rats treated with L-Dex or H-Dex compared with the VILI group (P<0.05 and P<0.01, respectively). Moreover, compared with the rats treated with VILI+H-Dex and VILI+L-Dex, there was a significant increase in cell death in the VILI+H-Dex+PD and VILI+L-Dex+PD groups, respectively (P<0.05 for both; Fig. 4). Additionally, there was no significant difference in cell death between the Sham group and the PD group.
Figure 4.
Dex reduces alveolar epithelial cell death. (A) PI-positive cells (red) and TUNEL-positive cells (green) were assessed by immunofluorescence staining; Hoechst 33342 (blue). Magnification, ×400; scale bar, 20 µm. (B) Apoptosis was quantitatively observed with TUNEL staining. (C) The quantification of necrosis using PI staining in each tested group. Data are presented as the mean ± SD (n=6). **P<0.01 vs. Sham; ##P<0.01 vs. VILI; &P<0.05 VILI+L-Dex+PD vs. VILI+L-Dex group; $$P<0.01 VILI+H-Dex+PD vs VILI+H-DexDex, dexmedetomidine; H-Dex, high dose Dex at 10 µg/kg; L-Dex, low dose Dex at 1 µg/kg; PD, PD98059; VILI, ventilator-induced lung injury.
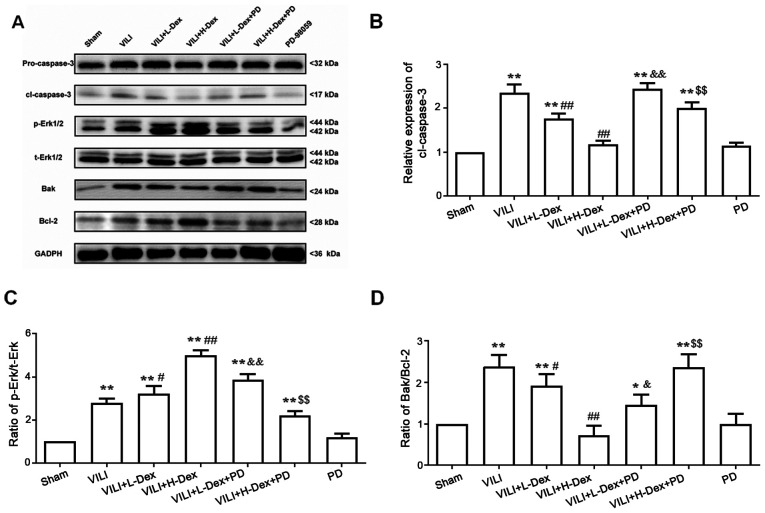
Dex may inhibit alveolar epithelial cell apoptosis and decrease the Bak/Bcl-2 ratio via ERK1/2 activation
To further understand the mechanism of Dex on cell death, the protein expression levels of Bak, Bcl-2, pro-caspase-3 and cleaved caspase-3 in the lung tissues were detected by western blotting (Fig. 5). The expression levels of Bak/Bcl-2 and cleaved caspase-3 significantly increased in the VILI, VILI+L-Dex, VILI+H-Dex, VILI+L-Dex+PD and VILI+H-Dex+PD groups compared with the Sham group (P<0.05; Fig. 5), whereas compared with the VILI group, L-Dex and H-Dex significantly reduced the expression levels of apoptotic proteins Bak/Bcl-2 and cleaved caspase-3. PD98059 co-treatment largely reversed the Bak/Bcl-2 and cleaved caspase-3 in the VILI+L-Dex+PD and VILI+H-Dex+PD groups compared with VILI+L-Dex and VILI+H-Dex. Moreover, there was no difference in pro-caspase-3 expression in each group.
Figure 5.
Dex may inhibit alveolar epithelial cell apoptosis and decrease the Bak/Bcl-2 ratio through ERK1/2 activation. (A) Representative western blotting images of pro- and cl-caspase-3, p-ERK1/2, t-ERK1/2, Bak and Bcl-2 in the lung. The semi-quantified protein expression level of (B) cl-caspase-3, (C) p-ERK/t-ERK and (D) Bak/Bcl-2. Data are presented as the mean ± SD (n=6). *P<0.05, **P<0.01 vs. Sham; #P<0.05, ##P<0.01 vs. VILI; &P<0.05, &&P<0.01 VILI+L-Dex+PD vs. VILI+L-Dex group; $$P<0.01 VILI+H-Dex+PD vs VILI+H-Dex. Cl, cleaved; Dex, dexmedetomidine; H-Dex, high dose Dex at 10 µg/kg; L-Dex, low dose Dex at 1 µg/kg; p-, phosphorylated; PD, PD98059; t-, total; VILI, ventilator-induced lung injury.
To investigate whether the antiapoptotic and anti-inflammatory effects of Dex were associated with the ERK1/2 pathway, PD98059 was administered prior to Dex treatment. Western blot analysis showed that the ratio of p-ERK/total (t)-ERK (which included phosphorylated and dephosphorylated ERK) significantly increased in VILI model rats compared with those in the Sham group (P<0.05; Fig. 5C). However, further analysis of the results indicated that the p-ERK/t-ERK ratio was significantly higher in the VILI+L-Dex and VILI+H-Dex group compared with the VILI group (P<0.05 and P<0.01, respectively; Fig. 5C). PD98059 co-treatment largely reversed the p-ERK/t-ERK ratio in the VILI+L-Dex+PD and VILI+H-Dex+PD group compared with VILI+L-Dex and VILI+H-Dex, respectively (both P<0.05; Fig. 5C).
Discussion
MV is a life-saving therapy for ARDS and patients with acute respiratory failure, such as acute lung injury, and is critical in general anesthesia (12). However, excessive ventilation can exacerbate pre-existing lung damage and damage healthy lungs, this process is known as VILI (22,23). The mechanisms of VILI involve atelectrauma, barotrauma, biotrauma and volutrauma (24), although there are numerous factors that have been considered as possible triggers for VILI. Based on a previous study (20), a rat VILI model using the HVT-MV method was successfully established in the present study.
Dex has a high affinity for α2 adrenoceptor and it is widely used in critically ill patients (at 1–1.5 µg/kg/h) owing to its sedative effects without respiratory inhibition (25,26). Numerous studies have also reported the anti-inflammatory effects of Dex by reducing the expression of inflammatory factors in certain specific cases (27–29). Considering previous literature reports and clinical dose analysis (11,12), two doses of Dex were examined in the present study. The data showed that high doses of Dex at 10 µg/kg/h significantly decreased expression of the inflammation-related cytokines IL-1β, IL-6 and TNF-α, W/D ratios and pathological changes in the VILI model rats. Several reports have demonstrated that Dex reduces the apoptosis of various parenchymal and epithelial cells following inflammation or ischemia (30–33). Similarly, H-Dex was found to significantly reduce alveolar epithelial cell apoptosis. The present results suggested that Dex may reduce lung injury via suppressing inflammatory responses and cell death. These protective effects may, in part, be due to α2 adrenoceptors.
In the present study, p-ERK1/2 expression was significantly increased in the lung tissue exposed to VILI or VILI+Dex. Moreover, Dex pretreatment appeared to induce more phosphorylation of ERK1/2. In addition, VILI treatment was demonstrated to induce a significant increase in cell death, whereas Dex pretreatment helped to attenuate these effects. However, when the phosphorylation of ERK1/2 was inhibited by PD98059, the protective effects of Dex appeared to be largely weakened. The neuroprotective role of Dex against cerebral ischemic injury may function via phosphorylation of ERK1/2 or the MAPK/ERK pathway (34,35). Similarly, the present study indicated that Dex pretreatment may protect alveolar epithelial cells against VILI-mediated effects through ERK1/2 activation.
A previous study has demonstrated that the activation of ERK1/2 may inhibit the NF-κB signaling pathway, which results in a reduction in the expression levels of inflammatory factors (for example, IL-1β, IL-6 and TNF-α) and apoptotic proteins, such as caspase-3 and Bak, thereby reducing hypoxia-induced damage (36). The present study results indicated that Dex reduced the expression levels of Bak, Bcl-2 and cleaved caspase-3, and decreases the secretion of inflammatory cytokines. However, these protective effects also partially following with the administration of PD98059. It has also been reported that activation of the ERK1/2 signal pathway may regulate the expression of aquaporins (37), in the present study, Dex reduced the ratio of W/D lung tissue weight, possibly by regulating the expression of aquaporins and activating ERK1/2. A study that compared the gene expression characteristics of lung injury in rodents and humans have found that conserved pathways, including MEK1/2-ERK1/2, are potential targets against the injury pathway (38). The MEK1/2-ERK1/2 pathway has an important role in inflammatory responses. It can be stimulated by signals downstream of Ras and Raf, and extracellular stimuli, such as growth factors and cytokines (39). PD98059 binds inactive enzymes of MEK1 and MEK2, which prevents their activation by Raf kinase, thereby inhibiting ERK1/2 activation (40). U0126 is known to directly inhibit the catalytic activity of active MEK1 and block MEK1/2 activity; U0126 primarily inhibits the phosphorylation of ERK (41). This study focused on ERK1/2 in the MEK/ERK pathway and demonstrated that PD98059 had a greater effect compared with U0126 in preliminary experiments, thus, PD98059 was chosen as an upstream inhibitor in this study.
In summary, the present study emphasized the protective effects of Dex against VILI and the role of Dex in the treatment of VILI. However, this study was still in its preliminary stage and further in vitro and in vivo studies of molecular mechanisms and signaling pathways are necessary to confirm these results.
Supplementary Material
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
CHZ and SQS conceptualized the study. CHZ, JY, BQW, YN, LW and SQS performed the experiments and collected the data. CHZ and JY analyzed the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All study procedures and animal handling were approved by the Animal Review Board of Cangzhou Central Hospital (Cangzhou, China; approval no. 2015-009-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ball L, Dameri M, Pelosi P. Best Practice and Research Clinical Anaesthesiology. Vol. 29. Elsevier; Amsterdam: 2015. Modes of mechanical ventilation for the operating room; pp. 285–299. [DOI] [PubMed] [Google Scholar]
- 2.Hartmann EK, Boehme S, Bentley A, Duenges B, Klein KU, Elsaesser A, Baumgardner JE, David M, Markstaller K. Influence of respiratory rate and end-expiratory pressure variation on cyclic alveolar recruitment in an experimental lung injury model. Crit Care. 2012;16:R8. doi: 10.1186/cc11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocco PR, Dos Santos C, Pelosi P. Pathophysiology of ventilator-associated lung injury. Curr Opin Anaesthesiol. 2012;25:123–130. doi: 10.1097/ACO.0b013e32834f8c7f. [DOI] [PubMed] [Google Scholar]
- 4.Usatyuk PV, Natarajan V. Hydroxyalkenals and oxidized phospholipids modulation of endothelial cytoskeleton, focal adhesion and adherens junction proteins in regulating endothelial barrier function. Microvasc Res. 2012;83:45–55. doi: 10.1016/j.mvr.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank JA, Parsons PE, Matthay MA. Pathogenetic significance of biological markers of ventilator-associated lung injury in experimental and clinical studies. Chest. 2006;130:1906–1914. doi: 10.1378/chest.130.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheiermann J, Klinman DM. Suppressive oligonucleotides inhibit inflammation in a murine model of mechanical ventilator induced lung injury. J Thorac Dis. 2016;8:2434–2443. doi: 10.21037/jtd.2016.08.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko YA, Yang MC, Huang HT, Hsu CM, Chen LW. NF-κB activation in myeloid cells mediates ventilator-induced lung injury. Respir Res. 2013;14:69. doi: 10.1186/1465-9921-14-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dent BT, Aarnes TK, Wavreille VA, Lakritz J, Lerche P, KuKanich B, Riccó Pereira CH, Bednarski RM. Pharmacokinetics and pharmacodynamic effects of oral transmucosal and intravenous administration of dexmedetomidine in dogs. Am J Vet Res. 2019;80:969–975. doi: 10.2460/ajvr.80.10.969. [DOI] [PubMed] [Google Scholar]
- 9.Ma D, Hossain M, Rajakumaraswamy N, Arshad M, Sanders RD, Franks NP, Maze M. Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharmacol. 2004;502:87–97. doi: 10.1016/j.ejphar.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi T, Kurita A, Kobayashi K, Yamamoto K, Inaba H. Dose- and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats. J Anesth. 2008;22:221–228. doi: 10.1007/s00540-008-0611-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Zhang Z, Chen K, Zhang F, Peng M, Wang Y. Dexmedetomidine regulates inflammatory molecules contributing to ventilator-induced lung injury in dogs. J Surg Res. 2014;187:211–218. doi: 10.1016/j.jss.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Sun X, Yang X, Hou Y, Yu X, Wang Y, Wu J, Liu D, Wang H, Yu J, et al. Dexmedetomidine reduces ventilator-induced lung injury (VILI) by inhibiting Toll-like receptor 4 (TLR4)/nuclear factor (NF)-κB signaling pathway. Bosn J Basic Med Sci. 2018;18:162–169. doi: 10.17305/bjbms.2018.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 14.Gong G, Yuan L, Cai L, Ran M, Zhang Y, Gong H, Dai X, Wu W, Dong H. Tetramethylpyrazine suppresses transient oxygen-glucose deprivation-induced connexin32 expression and cell apoptosis via the ERK1/2 and p38 MAPK pathway in cultured hippocampal neurons. PLoS One. 2014;9:e105944. doi: 10.1371/journal.pone.0105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Jiang B, Zhu N, Tao M, Jun Y, Chen X, Wang Q, Luo C. Mitotic checkpoint kinase Mps1/TTK predicts prognosis of colon cancer patients and regulates tumor proliferation and differentiation via PKCα/ERK1/2 and PI3K/Akt pathway. Med Oncol. 2019;37:5. doi: 10.1007/s12032-019-1320-y. [DOI] [PubMed] [Google Scholar]
- 16.Long ME, Gong KQ, Eddy WE, Volk JS, Morrell ED, Mikacenic C, West TE, Skerrett SJ, Charron J, Liles WC, et al. MEK1 regulates pulmonary macrophage inflammatory responses and resolution of acute lung injury. JCI Insight. 2019;4:e132377. doi: 10.1172/jci.insight.132377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyagi T, Tobe Y. Dexmedetomidine improves the histological and neurological outcomes 48 h after transient spinal ischemia in rats. Brain Res. 2014;1566:24–30. doi: 10.1016/j.brainres.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Sanders RD, Giombini M, Ma D, Ohashi Y, Hossain M, Fujinaga M, Maze M. Dexmedetomidine exerts dose-dependent age-independent antinociception but age-dependent hypnosis in Fischer rats. Anesth Analg. 2005;100:1295–1302. doi: 10.1213/01.ANE.0000149595.41576.B3. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Zhou S, Wang F, Zhou Y, Sheng S, Qi D, Huang JH, Wu E, Lv Y, Huo X. Hepatoprotective effects of limb ischemic post-conditioning in hepatic ischemic rat model and liver cancer patients via PI3K/ERK pathways. Int J Biol Sci. 2018;14:2037–2050. doi: 10.7150/ijbs.28435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li LF, Liao SK, Ko YS, Lee CH, Quinn DA. Hyperoxia increases ventilator-induced lung injury via mitogen-activated protein kinases: A prospective, controlled animal experiment. Crit Care. 2007;11:R25. doi: 10.1186/cc5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sammons MJ, Raval P, Davey PT, Rogers D, Parsons AA, Bingham S. Carrageenan-induced thermal hyperalgesia in the mouse: Role of nerve growth factor and the mitogen-activated protein kinase pathway. Brain Res. 2000;876:48–54. doi: 10.1016/s0006-8993(00)02596-8. [DOI] [PubMed] [Google Scholar]
- 22.Biehl M, Kashiouris MG, Gajic O. Ventilator-induced lung injury: Minimizing its impact in patients with or at risk for ARDS. Respir Care. 2013;58:927–937. doi: 10.4187/respcare.02347. [DOI] [PubMed] [Google Scholar]
- 23.Cortjens B, Royakkers AA, Determann RM, van Suijlen JD, Kamphuis SS, Foppen J, de Boer A, Wieland CW, Spronk PE, Schultz MJ, et al. Lung-protective mechanical ventilation does not protect against acute kidney injury in patients without lung injury at onset of mechanical ventilation. J Crit Care. 2012;27:261–267. doi: 10.1016/j.jcrc.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Ye L, Zeng Q, Dai H, Zhang W, Wang X, Ma R, Hong X, Zhao C, Pan L. Endoplasmic reticulum stress is involved in ventilator-induced lung injury in mice via the IRE1α-TRAF2-NF-κB pathway. Int Immunopharmacol. 2020;78:106069. doi: 10.1016/j.intimp.2019.106069. [DOI] [PubMed] [Google Scholar]
- 25.Cruickshank M, Henderson L, MacLennan G, Fraser C, Campbell M, Blackwood B, Gordon A, Brazzelli M. Alpha-2 agonists for sedation of mechanically ventilated adults in intensive care units: a systematic review. Health Technol Assess. 2016;20:v–xx, 1–117. doi: 10.3310/hta20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shehabi Y, Howe BD, Bellomo R, Arabi YM, Bailey M, Bass FE, Bin Kadiman S, McArthur CJ, Murray L, Reade MC, et al. ANZICS Clinical Trials Group and the SPICE III investigators Early sedation with dexmedetomidine in critically ill patients. N Engl J Med. 2019;380:2506–2517. doi: 10.1056/NEJMoa1904710. [DOI] [PubMed] [Google Scholar]
- 27.Gao J, Sun Z, Xiao Z, Du Q, Niu X, Wang G, Chang YW, Sun Y, Sun W, Lin A, et al. Dexmedetomidine modulates neuroinflammation and improves outcome via alpha2-adrenergic receptor signaling after rat spinal cord injury. Br J Anaesth. 2019;123:827–838. doi: 10.1016/j.bja.2019.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng F, Yan FF, Liu YP, Cong Y, Sun KF, He XM. Dexmedetomidine inhibits the NF-κB pathway and NLRP3 inflammasome to attenuate papain-induced osteoarthritis in rats. Pharm Biol. 2019;57:649–659. doi: 10.1080/13880209.2019.1651874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou XY, Liu J, Xu ZP, Fu Q, Wang PQ, Zhang HA. Dexmedetomidine inhibits the lipopolysaccharide-stimulated inflammatory response in microglia through the pathway involving TLR4 and NF-κB. Kaohsiung J Med Sci. 2019;35:750–756. doi: 10.1002/kjm2.12112. [DOI] [PubMed] [Google Scholar]
- 30.Xie Y, Guo C, Liu Y, Shi L, Yu J. Dexmedetomidine activates the PI3K/Akt pathway to inhibit hepatocyte apoptosis in rats with obstructive jaundice. Exp Ther Med. 2019;18:4461–4466. doi: 10.3892/etm.2019.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhai M, Liu C, Li Y, Zhang P, Yu Z, Zhu H, Zhang L, Zhang Q, Wang J, Wang J. Dexmedetomidine inhibits neuronal apoptosis by inducing Sigma-1 receptor signaling in cerebral ischemia-reperfusion injury. Aging (Albany NY) 2019;11:9556–9568. doi: 10.18632/aging.102404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J, Zheng S, Yang N, Chen B, He G, Zhu T. Dexmedetomidine inhibits apoptosis and expression of COX-2 induced by lipopolysaccharide in primary human alveolar epithelial type 2 cells. Biochem Biophys Res Commun. 2019;517:89–95. doi: 10.1016/j.bbrc.2019.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Cui J, Zhao H, Wang C, Sun JJ, Lu K, Ma D. Dexmedetomidine attenuates oxidative stress induced lung alveolar epithelial cell apoptosis in vitro. Oxid Med Cell Longev. 2015;2015:358396. doi: 10.1155/2015/358396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y, Peng XH, Li X, Luo GP, Wu MF. Neuroprotective role of dexmedetomidine pretreatment in cerebral ischemia injury via ADRA2A-mediated phosphorylation of ERK1/2 in adult rats. Exp Ther Med. 2018;16:5201–5209. doi: 10.3892/etm.2018.6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu Z, Lu P, Wang K, Zhao X, Li Q, Wen J, Zhang H, Li R, Wei H, Lv Y, et al. Dexmedetomidine inhibits neuroinflammation by altering microglial M1/M2 polarization through MAPK/ERK pathway. Neurochem Res. 2020;45:345–353. doi: 10.1007/s11064-019-02922-1. [DOI] [PubMed] [Google Scholar]
- 36.Jin H, Yu J. Lidocaine protects H9c2 cells from hypoxia-induced injury through regulation of the MAPK/ERK/NF-κB signaling pathway. Exp Ther Med. 2019;18:4125–4131. doi: 10.3892/etm.2019.8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen Q, Ma X, Hua Y, Chen M, Wang Y, Zhou Q, Ye W, Zhu X. Aquaporin 3 expression induced by salvia miltiorrhiza via ERK1/2 signal pathway in the primary human amnion epithelium cells from isolated oligohydramnios. Curr Mol Med. 2016;16:312–319. doi: 10.2174/1566524016666160225154304. [DOI] [PubMed] [Google Scholar]
- 38.Sweeney TE, Lofgren S, Khatri P, Rogers AJ. Gene expression analysis to assess the relevance of rodent models to human lung injury. Am J Respir Cell Mol Biol. 2017;57:184–192. doi: 10.1165/rcmb.2016-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caunt CJ, Sale MJ, Smith PD, Cook SJ. MEK1 and MEK2 inhibitors and cancer therapy: The long and winding road. Nat Rev Cancer. 2015;15:577–592. doi: 10.1038/nrc4000. [DOI] [PubMed] [Google Scholar]
- 40.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Zhang H, Liu E, Xu CB, Zhang Y. Homocysteine regulates endothelin type B receptors in vascular smooth muscle cells. Vascul Pharmacol. 2016;87:100–109. doi: 10.1016/j.vph.2016.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.