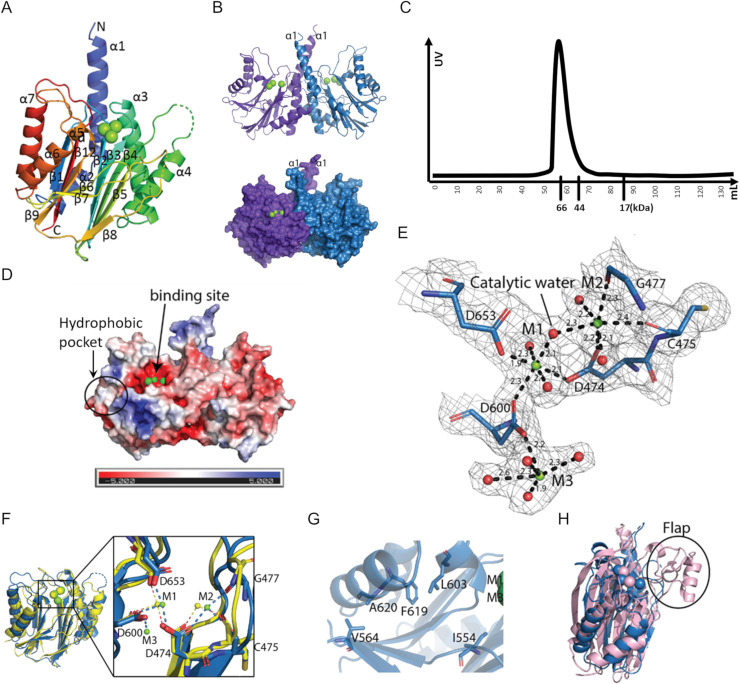
Fig 5. The SiaA-PP2C structure (PDB ID: 6K4E).
(A) “Rainbow” representation of the SiaA-PP2C monomer. The N-terminus of SiaA-PP2C is coloured in blue and the C-terminus in red, with secondary structures labelled. The three Mg2+ ions identified at the active site are represented as green spheres. (B) The SiaA-PP2C dimer displayed in cartoon (top) or surface (bottom). Monomer A is in purple and B in blue. (C) Size-exclusion chromatography of SiaA-PP2C. Elution volumes of markers are labelled below X-axis. (D) APBS surface electrostatic potential of SiaA-PP2C homodimer in the same orientation as in panel B. (E) Close-up view of the active site of the PP2C-like phosphatase domain of SiaA-PP2C with conserved acidic residues in the active site shown as sticks and labelled. An electron density map with Fourier coefficients 2FO-FC is overlaid at a level of 1σ above the mean. The Mg2+ ions are shown as green spheres and the oxygen atoms of the coordinating water molecules are displayed as red spheres. M1 and M2 are both coordinated by oxygen atoms from three residues and three water molecules in an octahedral manner. Distances between the metal ions and the coordinating atoms are indicated. (F) Overlay of SiaA_PP2C monomer B (blue) and the phosphatase domain of Rv1364C (yellow). Mg2+ ions M1 and M2 of SiaA-PP2C shown as green spheres overlap with Mn2+ ions of Rv1643C (yellow spheres). The α1 and α2 helices of SiaA-PP2C are not displayed for clarity. (inset) Magnified view of the active site. Metal coordinating residues of SiaA-PP2C are labelled. (G) Residues of the hydrophobic pocket shown as sticks. (H) Superposition of SiaA monomer B (blue) and human PPM1A (light pink; PDB code 6B67-A). The Flap sub-domain of human PPM1A (absent in SiaA-PP2C) is circled.