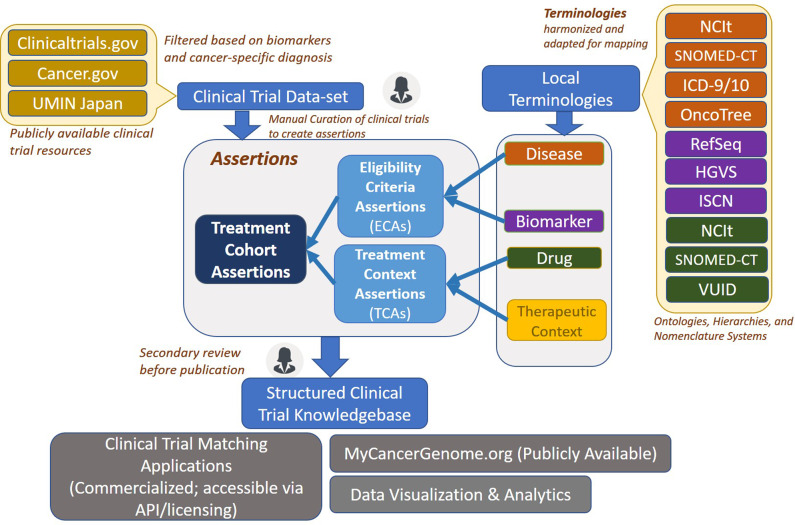
Figure 1.
Clinical trial model and workflow schematic. This high-level schematic describes the curation model components and workflow. Clinical trial documents are pulled into the clinical trial dataset from publicly available sources. Using the web-based interface, a curator creates structured assertions for trials. This is done using the terminologies and concept groups available in the data model. A single clinical trial can be broken down into multiple individual treatment cohort assertions (TCAs), each corresponding to a separate treatment arm. Once the assertions are created, they undergo a secondary manual review before being published into the clinical trial knowledge base. This knowledge base is utilized for clinical trial matching, display on My Cancer Genome website, as well as for multiple downstream applications.