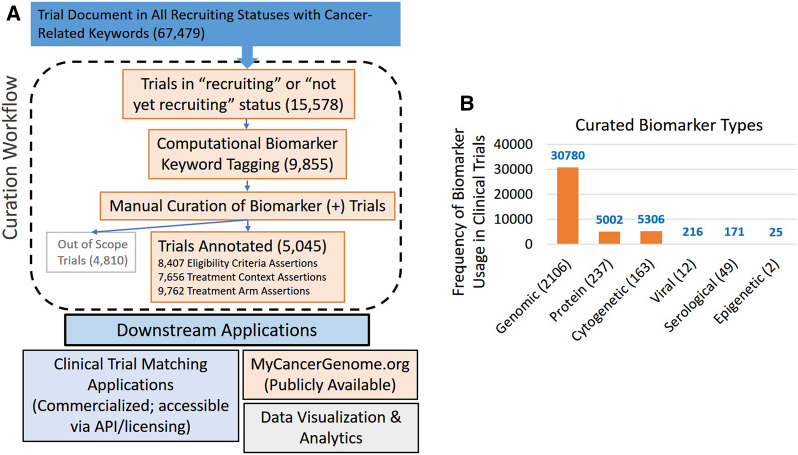
Figure 3.
Clinical trial curation workflow and results. The figure above shows (A) a broad overview of the curation workflow. There are currently 67 479 cancer-related clinical trials loaded into the system (as of 10/30/2019). Of these, 15 578 were found to have a status of recruiting or not-yet-recruiting. Of these, 9855 trials were automatically flagged for manual review as possibly containing biomarker key words. According to the curation SOP, of the 9855 manually reviewed clinical trials, 5045 met criteria for manual curation of disease-biomarker eligibility criteria and treatment context. A total of 4810 trials were considered out of scope based on the curation SOP. The trials that had a biomarker-driven eligibility criterion were curated. To date, we have manually curated and created structured annotations for 5045 clinical trials. A detailed copy of the SOP is provided in the Supplementary Material. Trials included for manual curation have a recruiting status of “Recruiting” or “Not yet recruiting,” that are (i) interventional (ii) directed toward treating cancer (not for treating side-effects or toxicities caused by cancer treatments), and (iii) contain biomarker-driven eligibility criteria (patient’s tumor is required to have a specific biomarker to enroll on the trial). (B) the different biomarker type supported by the system for clinical trial curation. Genomic biomarker makes up the largest category followed by protein, cytogenetic, viral, serological, and epigenetic-related biomarkers. The numbers in parentheses on the X-axis indicate the actual number of defined concepts in each category, while the instances of cumulative use across clinical trial curations are shown on the y-axis. The curated trial dataset (n = 5045) was used to calculate these numbers.