Fig. 4.
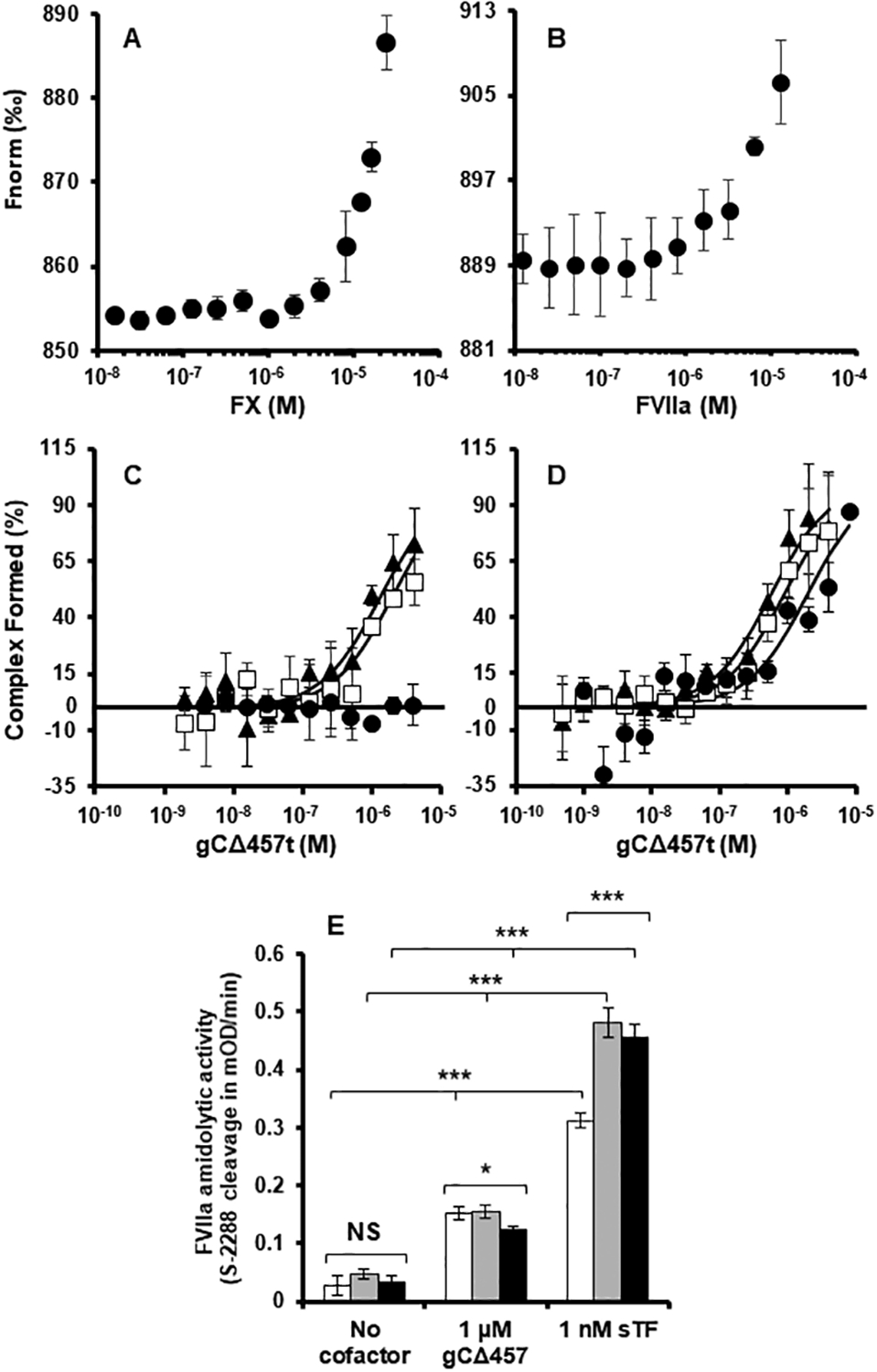
FX enhances the interaction between gCΔ457t and FVIIa. MST traces demonstrating weak associations of (A) FX and (B) FVIIa with gCΔ457t non-covalently labeled with RED-tris-NTA in the absence of membrane associations. (C) MST was also used to follow gCΔ457t binding to fluorescent FVIIa-R (2 nM) in the presence of PCPS (●), NiPC (◻) or NiPCPS (▲) (50 μM) and benzamidine to inhibit FVIIa. (D) Similar to (C) but with the inclusion of FX (30 nM). (E) Effects on FVIIa catalytic activity was assessed by following FVIIa (2 nM) cleavage of chromogenic substrate S-2288 in the presence of gCΔ457t (1 μM) or sTF-His (1 nM) with calcium (5 mM) and PCPS (white bars), NiPC (grey bars) or NiPCPS (black bars) (50 μM). (All graphs: n ≥ 3 ± SEM). P values are provided as determined by 1-way ANOVA. * P < 0.05, *** P < 0.001, P ≥ 0.05 was not significant (NS).
