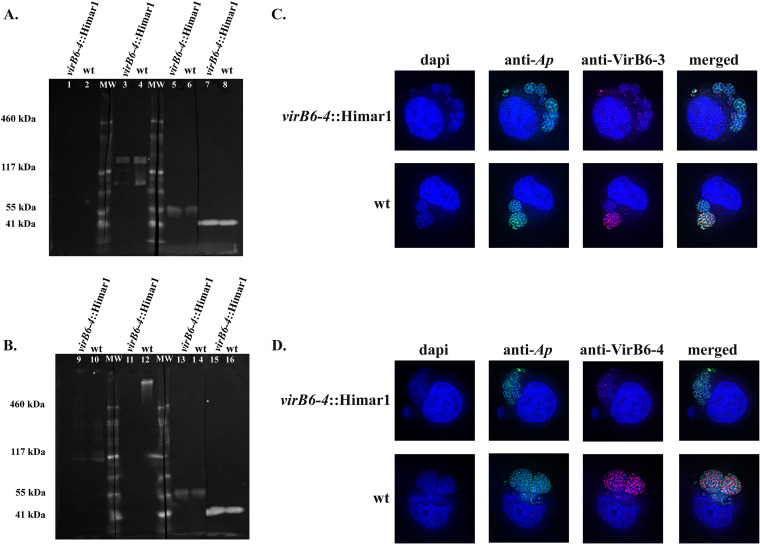
FIG 6.
Synthesis of VirB6-4 and VirB6-3 is disrupted in the virB6-4::Himar1 mutant. Equal amounts of proteins from host cell purified virB6-4::Himar1 and wt A. phagocytophilum were separated by Tris acetate polyacrylamide gel electrophoresis. (A) PVDF membranes of transferred proteins were reacted with rabbit preimmune serum (lanes 1 and 2), rabbit anti-VirB6-3 serum (lanes 3 and 4), and dog preimmune serum (lanes 5 and 6). Polyclonal dog serum infected with A. phagocytophilum, with immune responses mainly targeting the MSP2/P44 surface protein, was used as a loading control to indicate equal loading of proteins from the virB6-4::Himar1 mutant and wt A. phagocytophilum (lanes 7 and 8). (B) PVDF membranes of transferred proteins were reacted with rabbit preimmune serum (lanes 9 and 10), rabbit anti-VirB6-4 serum (lanes 11 and 12), dog preimmune serum (lanes 13 and 14), and A. phagocytophilum-infected dog serum (loading control; lanes 15 and 16). (C and D) HL-60 cells infected with A. phagocytophilum virB6-4::Himar1 or the wt were fixed and viewed by indirect immunofluorescence microscopy to determine immunoreactivity with A. phagocytophilum-infected mouse serum (green) and rabbit anti-VirB6-3 (C, red) or anti-VirB6-4 (D, red) serum. Representative immunoblots were obtained from three independent experiments. Representative immunofluorescence assay images from several micrographs are shown.