Summary
Converting CO2 into value-added chemical fuels and functional materials by CO2 reduction reaction (CO2RR) is conducive to achieving a carbon-neutral energy cycle. However, it is still challenging to efficiently navigate CO2RR toward desirable products. Herein, we report a facile strategy to extend product species in borate-containing molten electrolyte at a positively shifted cathodic potential with a high current density (e.g. 100 mA/cm2), which can selectively electro-transform CO2 into desired products (either CO or solid carbon nanofibers, respectively reaching a high selectivity of ∼90%). The borates can act as a controller of electrolyte alkalinity to buffer the concentration of sequentially generated O2− during CO2RR, positively shifting the reduction potential of the captured CO2 and concurrently extending the product species. The sustainable buffering effect is available under CO2 atmosphere. Compared with borate-free electrolyte, the CO2 conversion efficiency is over three times higher, while the electrolysis energy consumption is decreased by over 40%.
Subject Areas: Chemical Engineering, Electrochemistry, Industrial Chemistry
Graphical Abstract

Highlights
-
•
The product selectivity of CO2 reduction reaction was highly tunable
-
•
Extending product species via regulating electrolyte alkalinity was achieved
-
•
Borate-assisted oxygen removal was conducive to produce CO2-derived CNFs
-
•
Lower energy consumption and higher CO2 conversion efficiency were achieved
Chemical Engineering; Electrochemistry; Industrial Chemistry
Introduction
Ever since the industrial revolution, the atmospheric CO2 concentration has annually increased due to the anthropogenic emission (He et al., 2013). However, CO2 can also be regarded as an abundant C1 feedstock. Converting greenhouse gas CO2 into value-added chemical fuels and functional materials is both conducive to energy storage and CO2 mitigation, achieving a carbon-neutral energy cycle (MacDowell et al., 2010). Among various CO2 reduction reaction (CO2RR) approaches, electrochemical reduction processes are a simple strategy that can operate under environment-friendly conditions, especially the ones driven by renewable electricity sources (e.g., solar, wind) (Chen et al., 2018; Liu et al., 2019). Great attentions have been paid in various species of electrolytes in recent years, such as room temperature technologies (e.g., aqueous solutions, ionic liquids) (Chen and Mu, 2019; König et al., 2019; Rosen et al., 2011; Zhu et al., 2016), and high-temperature processes (e.g., solid oxide electrolysis cells and molten salt electrolytes) (Jiang et al., 2019; Song et al., 2019; Wu et al., 2018). Considering the fact that linear CO2 molecule is thermodynamically stable, it is still a great challenge to meet the energy demand for navigating CO2RR toward desired products more efficiently.
Very recently, due to high selectivity and excellent reaction kinetics, molten salt CO2 capture and electrotransformation (MSCC–ET) technology has attracted many attentions in converting high-flux CO2 into value-added products, including CO (Kaplan et al., 2010), carbon nanofibers (CNFs) (Douglas et al., 2017; Licht et al., 2016; Ren et al., 2015), hollow carbon spheres (Deng et al., 2017), ultrathin carbon sheet (Hu et al., 2015), and syngas or hydrocarbons were also achieved in the existence of water vapor (Wu et al., 2016). The high temperature process has some intrinsic advantages over the reduction near room temperature in terms of reaction thermodynamics, kinetics and product selectivity. The temperature of the system can be self-heated by Joule heat originated from the electrolysis process, where no extra energy input is needed to maintain the operating temperature for an industrial cell, making the energy balance more attractive (Sun et al., 2011; Sysoev et al., 2015). In addition, molten salts are also being used as effective media for commercial thermal energy storage heated by concentrated solar power in the world today, a large amount of CO2 can be reduced if a renewable electricity (e.g., solar, hydroelectric etc.) is applied, with a net CO2 conversion efficiency of 70–98%, depending on the sources of electricity (Jiang et al., 2019).
For conventional MSCC-ET systems, molten carbonates or carbonate-containing halides are the mainstream electrolytes to convert CO2 into solid carbon/CO, such as Li2CO3–Na2CO3–K2CO3 (Yin et al., 2013), LiCl–NaCl–Na2CO3, and CaCl2–CaCO3, etc (Ge et al., 2016b; Ijije et al., 2014a). Among those electrolytes, solid carbon materials are the most preferential CO2RR products, while electrolysis conditions are critical to gain specific microstructures (Deng et al., 2017, 2018). Although high-yield CO product was only observed in a selected molten electrolyte at a relatively high temperature (>900°C) exclusively using a Ti cathode (Kaplan et al., 2010), it is still challenging to achieve gaseous products under a mild condition due to thermodynamic and kinetic barriers as well as limitations of electrode alternatives (Chery et al., 2014). Moreover, the closed loop of the conventional molten salt CO2RR process is dependent on the supply of CO32− by carbonation of oxygen anions (O2−) with CO2 (known as acid gas), where the mass transfer of O2− is a rate-determining step (Gao et al., 2018; Deng et al., 2019). However, our recent works revealed that cathodic passivation effect, where solid oxides might easily precipitate on cathode, could favorably occur if the concentration (defined as alkalinity) of generated O2− in the electrolyte was oversaturated (Deng et al., 2019), severely increasing the cathodic polarization and retarding CO2RR kinetics (Gao et al., 2018). Hence, according to the state of the art, feasible pathways that can regulate product selectivity under mild operating conditions and with enhanced kinetics have not been reported yet.
Herein, we propose a facile strategy to manipulate the CO2RR selectivity at a positively shifted reduction potential by tuning alkalinity of electrolyte. Taking the conventional molten LiCl–Li2CO3 electrolyte for an example, it is demonstrated, for the first time, that adding LiBO2 into the electrolyte can thermodynamically change the reaction pathway compared to that in bare molten LiCl–Li2CO3, greatly enhancing CO2RR kinetics and extending product species at a lower cell voltage. Moreover, a tunable selectivity toward high-yield (∼90%) CO2-derived products (e.g., either CO or CNFs) at a relatively low temperature (e.g. <650°C) was also achieved. It is worth noting that the CO2 conversion efficiency was improved by over three times in molten LiCl–Li2CO3–LiBO2. To the best of our knowledge, the presented LiBO2-containing molten electrolyte is one of the most suitable electrolytes for high-flux CO2 capture and conversion regarding practical application.
Results and Discussion
Thermodynamic Characterizations of Alkalinity-Dependent CO2RR
Taking solid carbon and CO as CO2RR products for instances, the conventional conversion mechanisms of the captured CO2 (in the form of carbonate ions (CO32−)) in molten electrolytes can be described as follows (Deng et al., 2019; Yin et al., 2013):
Cathodic reactions:
| CO32− + 4e− = C + 3O2− | (Equation 1) |
| CO32− + 2e− = CO + 2O2− | (Equation 2) |
Anodic reaction on an inert anode:
| 2O2− – 4e− = O2 | (Equation 3) |
Supply of carbonate ions by CO2 absorption:
| O2− + CO2 = CO32− | (Equation 4) |
Overall reaction for CO2 reduction according to Equations 1, 2, 3, and 4:
| CO2 = C + O2 | (Equation 5) |
| CO2 = CO + 1/2O2 | (Equation 5a) |
Considering the conventional cathodic process as presented in Equation 1, our hypothesis is that converting sequentially generated O2− into other CO2 absorbing species could theoretically decrease O2− concentration (defined as alkalinity) in the electrolyte, which positively shifts reduction potential of absorbed CO2 (i.e. CO32−) according to the thermodynamic tendency based on Nernst equation, of which correlation between Nernstian potential (E) and alkalinity (aO2−) is shown in Figure 1A. As can be clearly seen, the theoretical reduction potential of CO32− producing carbon/CO can positively shift when the O2− concentration is decreased. More interestingly, the selectivity toward CO2RR product (such as carbon, CO) can also be regulated by controlling alkalinity of electrolyte during CO2 electrolysis, because CO is preferentially generated due to more positive reduction potential toward CO than that toward carbon when O2− concentration is greatly decreased (see CO-dominated region in Figure 1A).
Figure 1.
Thermodynamic Calculations and Electrochemical Measurements
(A) Correlation (E–pO) between reduction potential and oxygen anion concentration. .
(B) Thermodynamic calculations of possible cathodic reactions in molten LiCl–Li2CO3–LiBO2.
(C and D) Cyclic voltammograms in molten LiCl–Li2CO3 (C) and molten LiCl–Li2CO3–LiBO2 (D) with different scanning limits under Ar atmosphere at 550°C.
(E and F) Cyclic voltammograms in molten LiCl (E) and molten LiCl–1.25 mol% LiBO2 (F) under Ar atmosphere at 650°C.
From this perspective, we introduced BO2– (acting as O2− acceptor) into the electrolyte to form BO33− which showed excellent CO2 absorption capacity to regenerate BO2– under CO2 atmosphere (see Scheme 1) (Harada and Hatton, 2017), the mechanisms can be described as follows (Cherginets, 2005):
Scheme 1.
Comparative Schematics of Borate-Enhanced Molten Salt Electrolysis Technology
Conversion of electrochemically generated oxygen anions:
| BO2− + O2− ⇌ BO33− | (Equation 6) |
Supply of carbonate ions and oxygen anion acceptors:
| BO33− + CO2 ⇌ CO32− + BO2− | (Equation 7) |
According to Lux–Flood acid–base theory (Flood and Förland, 1947; Lux, 1939), O2− and BO2− (O2− acceptor) can be regarded as base and acid, respectively, therefore BO2−/BO33− is a conjugate acid–base pair as presented in Equation 6. Similar with buffer system theory in aqueous solution, the existence of BO2−/BO33− in molten salt can buffer O2− concentration, avoiding a sharp increase of alkalinity near cathode during CO2 electrolysis. To be specific, the sequentially released O2− during electroreduction of CO32− can spontaneously combine with BO2− to form BO33−, which can also act as CO2 absorbent to produce CO32− (electrochemical transformation precursor) and concurrently regenerate BO2− under CO2 atmosphere, setting up a self-supply closed loop for sustainable buffering effect and durable CO2RR.
To verify our hypothesis, molten LiCl–Li2CO3 (68.8:31.2 in molar ratio) was chosen as the electrolyte due to its simplex cation and relatively low melting point (517°C), LiBO2 was accordingly chosen as O2− acceptor to set up BO2−/BO33− conjugate acid–base pair. The comparative analysis of cyclic voltammetry (CV), potentiostatic/galvanostatic electrolysis and long-term electrolysis were conducted in molten LiCl–Li2CO3–LiBO2 compared to borate-free electrolyte using a homemade U-shape reactor equipped with an in situ gas analyzer (Figure S1).
As can be seen in Figure 1C, the cathodic peak c1 in borate-free electrolyte was attributed to the intrinsic reduction of CO32− (Equation 1), which is consisent with previous observations (Yin et al., 2013; Ge et al., 2016a). Remarkably, the CV in borate-containing electrolyte showed an extra broad reduction peak c2 (see Figure 1D), of which potential was more positive than intrinsic reduction of CO32− (peack c1). In accordance with our hypothesis, the appearance of peak c2 should be exclusively related to the positively shifted reduction of CO32−, which was attributed to the BO2− buffering effect toward as-formed O2− via Equations 8 and 9. Thermodynamic calculation (Figure 1B) as well as the CVs in LiCl (Figure 1E) and LiCl–LiBO2 (Figure 1F) also confirm this observation because the reduction potential of borate was much more negative than that of CO32−, indicating a promising electrochemial window of borate within the applied operating conditions.
Borate-assisted cathodic reactions:
| CO32− + 4e− + 3BO2− = C + 3BO33− | (Equation 8) |
| CO32− + 2e− + 2BO2− = CO + 2BO33− | (Equation 9) |
Oxidation of as-formed carbon during CV:
| C + 2O2− – 4e− = CO2 | (Equation 10) |
As can be seen in Figures 1C and 1D, it should be noted that the oxidation peak a1 in LiCl–Li2CO3–LiBO2, which was associated with sequentially released O2− during CV scanning via Equation 10 (Ijije et al., 2014b), accounted for a much smaller charge quantity ratio between anodic peak and cathodic peak compared to that in LiCl–Li2CO3 (see Table 1) within identical scanning limit, suggesting a greatly decreased O2− concentration due to the excellent buffering effect of BO2−/BO33− toward O2− via Equation 6. Similar observations were also found on different working electrodes, such as Mo, Ti, Cu, and graphite (Figure S2), showing an electrode-independent buffering effect related to BO2−/BO33−, which is consistent with our hypothesis according to Nernst equation as presented in Figure 1A.
Table 1.
The Charge Quantity Ratios between Anodic Peak (Qa) and Cathodic Peak (Qc) in Different Molten Electrolytes within Identical CV Scanning Potential Range
| Cathodic Limits | LiCl–Li2CO3 |
LiCl–Li2CO3–LiBO2 |
||||
|---|---|---|---|---|---|---|
| Qc1 (C) | Qa1 (C) | Qa1/Qc1 | Qc1+c2 (C) | Qa1 (C) | Qa1/Qc1+c2 | |
| −1.7 V | 0.664 | 0.134 | 0.202 | 0.485 | 0 | 0 |
| −1.8 V | 1.467 | 0.623 | 0.425 | 0.656 | 0.019 | 0.029 |
| −1.9 V | 2.722 | 1.553 | 0.571 | 1.270 | 0.301 | 0.237 |
Effects of Borate on Cathodic Polarization and Product Distribution
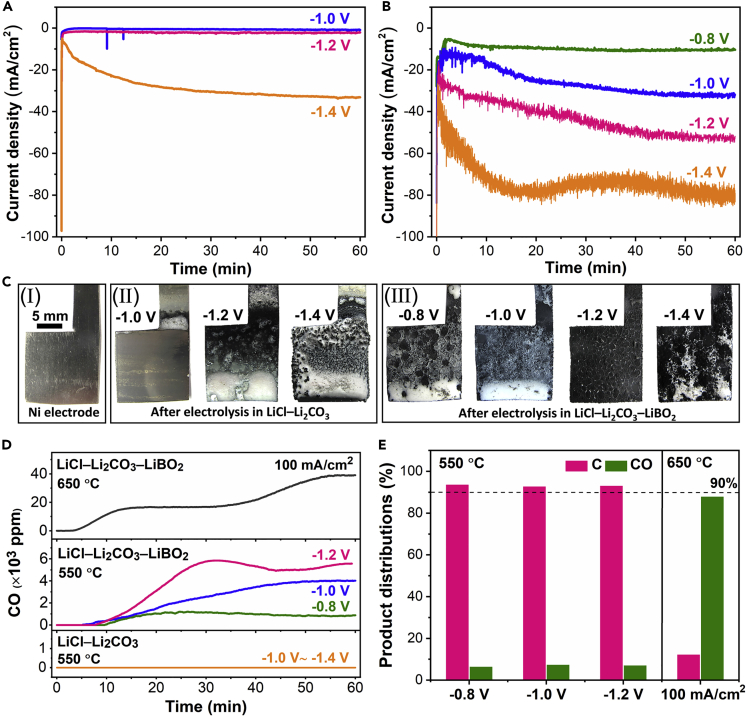
To elucidate the reactions occurring at different potentials and to reveal the critical role of LiBO2 toward product selectivity, the potentiostatic electrolysis was conducted on a plain Ni working electrode under Ar atmosphere. As can be seen in Figure 2A, neither obvious reduction current nor cathodic product (Figure 2C) was found in borate-free electrolyte below −1.0 V (vs. Ag/Ag2SO4). In the contrast, black deposits (i.e. carbon products, see Figure 2C) and noticeable reduction current (see Figure 2B) can be observed when applied potential was merely −0.8 V (vs. Ag/Ag2SO4) in borate-containing electrolyte. Specifically, the cathodic current density was over two times higher than that in LiCl–Li2CO3 at the same applied potential of −1.4 V (vs. Ag/Ag2SO4). Furthermore, the reduction potential was positively shifted by 400 mV to reach similar cathodic current density in borate-containing electrolyte compared to that in LiCl–Li2CO3 at −1.4 V (vs. Ag/Ag2SO4). Remarkably, the in-situ gas analyzer displayed that newly generated gaseous product species (i.e. CO, see Figure 2D) was commonly observed in borate-containing electrolyte during electrolysis, together with solid carbon products (see Figure 2C), which is clearly in accordance with our hypothesis and thermodynamic analysis. Whereas no CO but only solid carbon products were generated during the electrolysis within the investigated cathodic potential range (−1.0 to −1.4 V vs. Ag/Ag2SO4) in borate-free electrolyte, which is in good agreement with previous report (Ge et al., 2016a). Apparently, the distribution of CO2RR products in molten LiCl–Li2CO3–LiBO2 was also dependent on operating temperature and applied electrode potential (see Figure 2E), suggesting a highly tunable product selectivity toward CO2RR. It is worth-mentioning that our finding further revealed that the CO content was significantly increased (see Figure 2D) and the yield can reach as high as ∼90% at a high current density (e.g. 100 mA/cm2) by simply elevating electrolysis temperature (e.g. 650°C), where the current density for producing CO was at least 5 times higher than that in aqueous solution using noble metal catalysts (e.g. Au, Pd, <20 mA/cm2) (Gao et al., 2015; Hall et al., 2015; Zhu et al., 2019). Furthermore, the electrode-independent high-yield CO production presented in this work was achieved at a relatively low temperature (650°C), exhibiting more advantages than previously reported molten salt electrolysis technology, in which the electrolysis temperature was as high as 900°C and a Ti cathode was exclusively used (Kaplan et al., 2010).
Figure 2.
Potentiostatic Electrolysis and Product Distributions
(A and B) Current-time plots of potentiostatic electrolysis in (A) molten LiCl–Li2CO3 and (B) molten LiCl–Li2CO3–LiBO2 under Ar atmosphere at 550°C.
(C) Corresponding optical images of Ni electrode before and after potentiostatic electrolysis.
(D) Detected CO content during potentiostatic and galvanostatic electrolysis.
(E) Product distributions in molten LiCl–Li2CO3–LiBO2 under various conditions.
Considering that solid carbon is more preferentially obtained at 550°C (selectivity>90%), it is very interesting to find that nanostructures of carbon products can also be tailored in borate-containing electrolyte. To be specific, the SEM images of carbon products obtained in LiCl–Li2CO3–LiBO2 illustrate that CNFs were commonly observed within −0.8 ∼ −1.4 V at 550°C (see Figures 3A–3D), accounting for at least 30% under respective electrolysis condition, whereas carbon products obtained in borate-free electrolyte were completely micron-scale quasi-spherical particles (Figure 3E), suggesting that borate can help regulate one-dimensional-oriented growth for carbon nucleis. Specifically, Figure 3B shows that the high-content (>80%) CNFs with diameter of ∼80 nm were observed. Considering that oxygen atoms play significant roles on carbon nanostructures, the growth of CNFs might be attributed to the in situ deoxidation via a buffering effect (Equation 6) during carbon nucleation, triggering the reforming of as-formed carbon atoms because lower oxygen content (see Table 2) and higher graphitic crystallinity (see higher G band in Figure 3B) were observed for CNFs compared to those for quasi-spherical particles. The critical role of oxygen removal on CNFs growth has also been reported by other work (Moyer et al., 2020), in which fast O2 evolution originating from anodic process of O2− at anode (Equation 3) is conducive to the growth of CNFs/CNTs. The findings in this work suggest the similar effort of O2− removal; however, by a homogeneous process, where the borate in the electrolyte can help remove O2− generated near cathode via Equation 6. In addition, it should be pointed out that no boron (B) was found in the obtained carbon products according to the X-ray photoelectron spectrometry (XPS) result (Table 2), suggesting the excellent thermodynamic stability of borates in the applied potential range, which was consistent with the observation in the CVs (Figure 1F).
Figure 3.
SEM and Raman Characterization of Carbon Products
(A–D) SEM image of carbon products obtained under potentiostatic electrolysis at (A) −0.8 V, (B) −1.0 V, (C) −1.2 V and (D) −1.4 V in molten LiCl–Li2CO3–LiBO2.
(E) SEM images of carbon product obtained under potentiostatic electrolysis at −1.4 V in molten LiCl–Li2CO3.
(F) Raman spectrums of carbon products obtained in different molten electrolytes.
Table 2.
The XPS Analysis of Carbon Products Obtained under Potentiostatic Electrolysis in Different Molten Electrolytes
| Element (Atom%) | LiCl–Li2CO3–LiBO2 |
LiCl–Li2CO3 |
|||
|---|---|---|---|---|---|
| −0.8 V | −1.0 V | −1.2 V | −1.4 V | −1.4 V | |
| C | 94.06 | 96.03 | 95.68 | 95.51 | 83.25 |
| O | 5.52 | 3.97 | 4.10 | 4.25 | 14.34 |
| B | 0 | 0 | 0 | 0 | 0 |
| Ti | 0 | 0 | 0 | 0 | 0 |
| Ni | 0.42 | 0 | 0.22 | 0.24 | 2.41 |
The optimum conditions for producing high-yield either CO or CNFs were in good agreement with thermodynamic analysis (see Figures 1A and 1B), where elevating temperature and decreasing alkalinity of electrolyte can both give rise to a considerably high content of CO. Apparently, the performance that manipulating selectivity by tuning electrolyte alkalinity is also associated with borate concentration, which needs further investigation in the future.
Effects of Borate on Cathodic Passivation and CO2 Conversion Efficiency
Cathodic passivation effect:
| 2Li+ + O2− ⇌ Li2O (s) | (Equation 11) |
Poor CO2 capture by solid Li2O:
| Li2O (s) + CO2 (g) = Li2CO3 (s) | (Equation 12) |
As shown in Equation 11, cathodic passivation effect usually results from accumulation of solid oxides (e.g. Li2O) on cathode, of which solubility in molten electrolytes is limited (Gao et al., 2018), leading to an increasing cathodic polarization because of the undesirable electric resistance of covered oxides (Gao et al., 2018; Deng et al., 2019), which also retard both CO2 capture (Equation 12) and sequential electrotransformation. To further verify the positive effort inhibiting cathodic passivation effect when borate is added into the electrolyte, the galvanostatic electrolysis at a high cathodic current density (e.g. 260 mA/cm2) was carried out in molten LiCl–Li2CO3–LiBO2 and molten LiCl–Li2CO3 with identical electrolysis duration, respectively. It has been previously reported that Li2O content on raw cathode after electrolysis is an indicator to the degree of passivation effect, where higher current density can preferentially lead to more severe cathodic passivation effect while higher Li2O content on raw cathode can be observed (Gao et al., 2018). Therefore, after the electrolysis, the raw cathodic products were analyzed by X–ray diffraction spectroscopy (XRD) without any post-treatment. As shown in Figure 4A, the XRD patterns clearly show that cathodic passivation effect was greatly inhibited in borate-containing electrolyte, because a much lower relative intensity (ILi2O:Imelt) of Li2O was observed, which is consistent with our hypothesis that borate can remove O2− in the electrolyte via Equation 6.
Figure 4.
Effects of Borate on Cathodic Passivation and CO2 Conversion Efficiency
(A) XRD patterns of raw cathodic products after galvanostatic electrolysis at 260 mA/cm2 in different molten electrolytes under 550°C.
(B) Variations of cell voltage and outlet CO2 concentration during galvanostatic electrolysis at 23 mA/cm2 in different molten electrolytes under 550°C.
From the perspective of practical application, a mixed gas containing 14 vol% CO2 (the same CO2 content as flue gas) and Ar was bubbled into the melt. To gain a further insight into the efforts of borate buffer system on overall energy consumption, long-term working stability and CO2 conversion efficiency, the variations of cell voltage and outlet CO2 concentration during galvanostatic electrolysis were real-time monitored for 18 h. As can be seen in Figure 4B, the applied cell voltage for CO2RR in molten LiCl–Li2CO3–LiBO2 was 600 mV lower than that in molten LiCl–Li2CO3 under the same cathodic current density (e.g. 23 mA/cm2), merely accounting for approximately 60% of electrolysis energy consumption in molten borate-free electrolyte. Considering that small motive Li+ cations dominate electrical conductivity in Li-rich molten species (Katsumata et al., 1999a, 1999b), therefore, the influence of electrical conductivity in molten salt with and without LiBO2 might be marginal in this work because Li+ is the only cation in the selected molten electrolyte. Given the consideration above, the positively shifted cathodic potential and the inhibited cathodic passivation effect in borate-containing electrolyte should be responsible for the much lower applied cell voltage.
As shown in Figure 4B, the slightly fluctuated variation of cell voltage demonstrated a long-lasting operating stability for borate-assisted CO2RR. More importantly, according to the contrast of CO2 outlet concentration (Figure 4B), it is interesting to find that the CO2 conversion efficiency in molten LiCl–Li2CO3–LiBO2 was over three times higher than that in LiCl–Li2CO3. The increasing amount of CO2 absorbing species and inhibited cathodic passivation effect in borate-containing molten electrolyte should both be responsible for the lower CO2 outlet concentration. Specifically, for borate-free electrolyte, O2− acting as CO2 absorbent was sequentially generated during CO2RR via Equation 1, whereas cathodic passivation effect easily occurred when O2− was oversaturated (Equation 11), leading to precipitation of solid Li2O on cathodes together with poor CO2 absorption performance (Equation 12). By contrast, for borate-containing electrolyte, owing to the buffering effect of borates toward O2−, the cathodic passivation effect can be effectively inhibited due to the O2− removal by Equation 6, instead, a more soluble high-performance CO2 absorbing species (i.e. BO33−) was generated, which can regenerate BO2− and concurrently produce CO32− under CO2 atmosphere (Equation 7). Therefore, the CO2 absorption performance of overall CO2 absorbing species (BO33− and O2−) in borate-containing electrolyte was better than those (mainly O2−, solid Li2O) in borate-free electrolyte. In other words, the synergistic CO2 absorption in borate-containing electrolyte showed superior advantage regarding CO2 capture and conversion efficiency, achieving a three-time higher CO2 conversion efficiency under identical current density in comparison with borate-free electrolyte. The gradually decreased but eventually stable CO2 outlet concentration for borate-containing electrolyte (see Figure 4B) indicates that the acid–base equilibrium involving Equations 6, 7, 8, and 9 was proceeding during the electrolysis.
To gain more insights into the advantages of this work, a comparative analysis based on the benchmarking of molten salt CO2RR regarding cathodic potential, energy consumption and reaction conditions in different electrolytes is listed in Table S1. Remarkably, it should be pointed out that the presented borate-containing electrolyte in this work exhibited more positive onset potential related to CO2RR, suggesting a more favorable energy demand. More interestingly, the cathodic potential in borate-containing electrolyte was much more positive than other reported electrolysis systems, which can be clearly observed under identical operating condition (i.e. 35 mA/cm2, 550°C) using the same electrodes, indicating a higher energy efficiency. Accordingly, the both much lower cell voltage and energy consumption for producing solid carbon product by reducing 1 kg of CO2 further confirmed the benefits of CO2RR in borate-containing electrolyte. More importantly, with the assistance of borate, the extended gaseous product (i.e. CO) with a high faradaic efficiency of ∼90% was achieved, whilst the operating temperature and the applied cell voltage both were much lower than previously reported work under optimum conditions. Regarding positively shifted reduction potential, lower energy consumption and tunable product selectivity, achieving CO2RR in borate-containing electrolyte under mild operating conditions exhibits superior advantages than conventional borate-free processes.
The long-term stability of borate buffering system during CO2RR is the prerequisite in the viewpoint of practical application. Similar with classical acid–base buffering systems in aqueous solution, the buffering capacity of BO2−/BO33− pairs is dependent on the concentration of borates. For a given borate buffer concentration (e.g. 5 mol% LiBO2 in this work), the buffering effect toward base (O2−) might not work well if the as-formed O2− concentration was too high, which is related to the inharmonic electrolysis parameters (such as high current density or high cell voltage) because the amount of sequentially generated O2− during electrolysis is beyond the buffering capacity of BO2−/BO33− pairs. To ensure the stability of buffer system, it is also important to precisely control the applied cathodic polarization within the potential range of which borates may not be reduced. Considering that the sustainable supply of BO2− is available via CO2 absorption (Equation 7), therefore, the CO2 atmosphere and suitable operating temperature are other important factors contributing to sustaining BO2−/BO33− pairs. Given the considerations above, the long-term stability of borate-assisted electrolysis presented in this work can be guaranteed under CO2 atmosphere and at a moderate current density (e.g. 23 mA cm−2 in Figure 4B).
Conclusion
We demonstrate a facile strategy to manipulate CO2RR selectivity at a positively shifted reduction potential by regulating electrolyte alkalinity, which is associated with the buffering effect of borate. Specifically, the as-formed borate conjugate acid–base pair can effectively buffer the concentration of electrochemically generated O2− in the electrolyte, avoiding a sharp increase of alkalinity and concurrently inhibiting cathodic passivation effect during CO2RR. Owing to the borate buffering effect toward electrolyte alkalinity, a newly extended product species (i.e. CO) was achieved, of which selectivity (either CO or CNFs) was highly tunable by simply regulating electrolysis conditions. In particular, ∼90% CO can be obtained at a high current density (e.g. 100 mA/cm2). It was further revealed that introducing borate into the electrolyte is conducive to transforming pristine CO2-derived carbon quasi-spherical particles into CNFs. In comparison with CO2RR in borate-free electrolyte, the overall energy consumption of borate-assisted process can be decreased by 40%, while the CO2 conversion efficiency can be increased by 300%. The findings not only give clues to extending CO2RR product species under mild operating condition but also provide a feasible pathway to achieve carbon-neutral cycle at a higher energy efficiency.
Limitations of the Study
This work provides a borate-assisted strategy to navigate CO2RR pathway by controlling electrolyte alkalinity. Restrained by the cathodic limitation of borates, it is currently difficult to achieve CO2RR at a considerably higher current density within limited electrochemical window. Hence, a more electrochemically stable buffer system should be developed in the future.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dihua Wang (wangdh@whu.edu.cn).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The data that support the findings of this study are available from corresponding author upon reasonable request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was funded by National Natural Science Foundation of China (No. 21673162), China Postdoctoral Science Foundation (No. 2019M652708).
Author Contributions
L.H. and B.D. contributed equally. Conceptualization and Writing – Original Draft, L.H., B.D.; Formal Analysis, B.D., D.W., K.D.; Visualization, R.J., Y.D.; Methodology, L.H., B.D., D.W.; Funding Acquisition, D.W., B.D.
Declaration of Interests
The authors declare no competing interests.
Published: October 23, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101607.
Supplemental Information
References
- Chen K.-J., Yang Q.-Y., Sen S., Madden D.G., Kumar A., Pham T., Forrest K.A., Hosono N., Space B., Kitagawa S., Zaworotko M.J. Efficient CO2 removal for ultra-pure CO production by two hybrid ultramicroporous materials. Angew. Chem. Int. Ed. 2018;57:3332–3336. doi: 10.1002/anie.201706090. [DOI] [PubMed] [Google Scholar]
- Chen Y., Mu T. Conversion of CO2 to value-added products mediated by ionic liquids. Green. Chem. 2019;21:2544–2574. [Google Scholar]
- Cherginets V.L. Oxoacidity: reactions of oxo-compounds in ionic solvents. Elsevier; 2005. [Google Scholar]
- Chery D., Albin V., Lair V., Cassir M. Thermodynamic and experimental approach of electrochemical reduction of CO2 in molten carbonates. Int. J. Hydrogen Energy. 2014;39:12330–12339. [Google Scholar]
- Deng B., Gao M., Yu R., Mao X., Jiang R., Wang D. Critical operating conditions for enhanced energy-efficient molten salt CO2 capture and electrolytic utilization as durable looping applications. Appl. Energy. 2019;255:113862. [Google Scholar]
- Deng B., Mao X., Xiao W., Wang D. Microbubble effect-assisted electrolytic synthesis of hollow carbon spheres from CO2. J. Mater. Chem. A. 2017;5:12822–12827. [Google Scholar]
- Deng B., Tang J., Gao M., Mao X., Zhu H., Xiao W., Wang D. Electrolytic synthesis of carbon from the captured CO2 in molten LiCl–KCl–CaCO3: critical roles of electrode potential and temperature for hollow structure and lithium storage performance. Electrochim. Acta. 2018;259:975–985. [Google Scholar]
- Douglas A., Carter R., Muralidharan N., Oakes L., Pint C.L. Iron catalyzed growth of crystalline multi-walled carbon nanotubes from ambient carbon dioxide mediated by molten carbonates. Carbon. 2017;116:572–578. [Google Scholar]
- Flood H., Förland T. The acidic and basic properties of oxides. Acta Chem. Scand. 1947;1:592–604. doi: 10.3891/acta.chem.scand.01-0592. [DOI] [PubMed] [Google Scholar]
- Gao D., Zhou H., Wang J., Miao S., Yang F., Wang G., Wang J., Bao X. Size-dependent electrocatalytic reduction of CO2 over pd nanoparticles. J. Am. Chem. Soc. 2015;137:4288–4291. doi: 10.1021/jacs.5b00046. [DOI] [PubMed] [Google Scholar]
- Gao M., Deng B., Chen Z., Tao M., Wang D. Cathodic reaction kinetics for CO2 capture and utilization in molten carbonates at mild temperatures. Electrochem. Commun. 2018;88:79–82. [Google Scholar]
- Ge J., Hu L., Song Y., Jiao S. An investigation into the carbon nucleation and growth on a nickel substrate in LiCl-Li2CO3 melts. Faraday Discuss. 2016;190:259–268. doi: 10.1039/c5fd00217f. [DOI] [PubMed] [Google Scholar]
- Ge J., Wang S., Hu L., Zhu J., Jiao S. Electrochemical deposition of carbon in LiCl–NaCl–Na2CO3 melts. Carbon. 2016;98:649–657. [Google Scholar]
- Hall A.S., Yoon Y., Wuttig A., Surendranath Y. Mesostructure-induced selectivity in CO2 reduction catalysis. J. Am. Chem. Soc. 2015;137:14834–14837. doi: 10.1021/jacs.5b08259. [DOI] [PubMed] [Google Scholar]
- Harada T., Hatton T.A. Tri-lithium borate (Li3BO3); a new highly regenerable high capacity CO2 adsorbent at intermediate temperature. J. Mater. Chem. A. 2017;5:22224–22233. [Google Scholar]
- He M., Sun Y., Han B. Green carbon science: scientific basis for integrating carbon resource processing, utilization, and recycling. Angew. Chem. Int. Ed. 2013;52:9620–9633. doi: 10.1002/anie.201209384. [DOI] [PubMed] [Google Scholar]
- Hu L., Song Y., Ge J., Zhu J., Jiao S. Capture and electrochemical conversion of CO2 to ultrathin graphite sheets in CaCl2-based melts. J. Mater. Chem. A. 2015;3:21211–21218. [Google Scholar]
- Ijije H.V., Lawrence R.C., Siambun N.J., Jeong S.M., Jewell D.A., Hu D., Chen G.Z. Electro-deposition and re-oxidation of carbon in carbonate-containing molten salts. Faraday Discuss. 2014;172:105–116. doi: 10.1039/c4fd00046c. [DOI] [PubMed] [Google Scholar]
- Ijije H.V., Sun C., Chen G.Z. Indirect electrochemical reduction of carbon dioxide to carbon nanopowders in molten alkali carbonates: process variables and product properties. Carbon. 2014;73:163–174. [Google Scholar]
- Jiang R., Gao M., Mao X., Wang D. Advancements and potentials of molten salt CO2 capture and electrochemical transformation (MSCC-ET) process. Curr. Opin. Electrochem. 2019;17:38–46. [Google Scholar]
- Kaplan V., Wachtel E., Gartsman K., Feldman Y., Lubomirsky I. Conversion of CO2 to CO by electrolysis of molten lithium carbonate. J. Electrochem. Soc. 2010;157:B552–B556. [Google Scholar]
- Katsumata T., Suzuki N., Shibasaki M., Matsuo T. Electrical conductivity and nuclear magnetic resonance of molten lithium borates. ECS Proc. 1999;1999–41:383–391. [Google Scholar]
- Katsumata T., Suzuki N., Shibasaki M., Matsuo T. Electric field application to molten lithium borates. In: Ramachandran N., editor. Materials Research in Low Gravity II. 1999. pp. 242–249. [Google Scholar]
- König M., Vaes J., Klemm E., Pant D. Solvents and supporting electrolytes in the electrocatalytic reduction of CO2. iScience. 2019;19:135–160. doi: 10.1016/j.isci.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht S., Douglas A., Ren J., Carter R., Lefler M., Pint C.L. Carbon nanotubes produced from ambient carbon dioxide for environmentally sustainable lithium-ion and sodium-ion battery anodes. ACS Cent. Sci. 2016;2:162–168. doi: 10.1021/acscentsci.5b00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Lu X.F., Xiao J., Wang X., Lou X.W.D. Bi2O3 nanosheets grown on multi-channel carbon matrix to catalyze efficient CO2 electroreduction to HCOOH. Angew. Chem. Int. Ed. 2019;58:13828–13833. doi: 10.1002/anie.201907674. [DOI] [PubMed] [Google Scholar]
- Lux H. “Säuren” und “Basen” im Schmelzfluss: die Bestimmung der Sauerstoffionen-Konzentration. Z. für Elektrochemie Angew. Phys. Chem. 1939;45:303–309. [Google Scholar]
- MacDowell N., Florin N., Buchard A., Hallett J., Galindo A., Jackson G., Adjiman C.S., Williams C.K., Shah N., Fennell P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010;3:1645–1669. [Google Scholar]
- Moyer K., Zohair M., Eaves-Rathert J., Douglas A., Pint C.L. Oxygen evolution activity limits the nucleation and catalytic growth of carbon nanotubes from carbon dioxide electrolysis via molten carbonates. Carbon. 2020;165:90–99. [Google Scholar]
- Ren J., Li F.-F., Lau J., González-Urbina L., Licht S. One-pot synthesis of carbon nanofibers from CO2. Nano Lett. 2015;15:6142–6148. doi: 10.1021/acs.nanolett.5b02427. [DOI] [PubMed] [Google Scholar]
- Rosen B.A., Salehi-Khojin A., Thorson M.R., Zhu W., Whipple D.T., Kenis P.J.A., Masel R.I. Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials. Science. 2011;334:643–644. doi: 10.1126/science.1209786. [DOI] [PubMed] [Google Scholar]
- Song Y., Zhang X., Xie K., Wang G., Bao X. High-temperature CO2 electrolysis in solid oxide electrolysis cells: developments, challenges, and prospects. Adv. Mater. 2019;31:1902033. doi: 10.1002/adma.201902033. [DOI] [PubMed] [Google Scholar]
- Sun Z., Liu C., Lu G., Song X., Sun S., Sun Y., Yu J. Coupled thermoelectric model and effects of current fluctuation on thermal balance in magnesium electrolysis cell. Energy Fuels. 2011;25:2655–2663. [Google Scholar]
- Sysoev I.A., Ershov V.A., Kondrat’ev V.V. Method of controlling the energy balance of electrolytic cells for aluminum production. Metallurgist. 2015;59:518–525. [Google Scholar]
- Wu H., Ji D., Li L., Yuan D., Zhu Y., Wang B., Zhang Z., Licht S. A New technology for efficient, high yield carbon dioxide and water transformation to methane by electrolysis in molten salts. Adv. Mater. Technol. 2016;1:1600092. [Google Scholar]
- Wu W., Ding H., Zhang Y., Ding Y., Katiyar P., Majumdar P.K., He T., Ding D. 3D self-architectured steam electrode enabled efficient and durable hydrogen production in a proton-conducting solid oxide electrolysis cell at temperatures lower than 600 °C. Adv. Sci. 2018;5:1800360. doi: 10.1002/advs.201800360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Mao X., Tang D., Xiao W., Xing L., Zhu H., Wang D., Sadoway D.R. Capture and electrochemical conversion of CO2 to value-added carbon and oxygen by molten salt electrolysis. Energy Environ. Sci. 2013;6:1538–1545. [Google Scholar]
- Zhu D.D., Liu J.L., Qiao S.Z. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Adv. Mater. 2016;28:3423–3452. doi: 10.1002/adma.201504766. [DOI] [PubMed] [Google Scholar]
- Zhu S., Qin X., Wang Q., Li T., Tao R., Gu M., Shao M. Composition-dependent CO2 electrochemical reduction activity and selectivity on Au-Pd core-shell nanoparticles. J. Mater. Chem. A. 2019;7:16954–16961. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from corresponding author upon reasonable request.