Abstract
Background
This study aimed to review the activity of cytotoxic chemotherapy in patients with inflammatory myofibroblastic tumors (IMTs) treated at nine European sarcoma reference centers.
Materials and Methods
Patients of any age, with histologically proven IMT, treated with anthracycline‐based methotrexate plus/minus vinorelbine/vinblastine (MTX‐V) or other chemotherapeutic regimens between 1996 and 2018 were retrospectively reviewed. Diagnosis was confirmed at the local level by an expert pathologist. Response was retrospectively assessed by local investigators by RECIST v1.1. Progression‐free survival (PFS), relapse‐free survival (RFS), and overall survival (OS) were computed by Kaplan‐Meier method.
Results
Thirty‐eight patients were included. Twenty‐five patients (8 localized, 17 advanced disease) received an anthracycline‐based regimen; 21 were evaluable for response. Overall response rate (ORR) was 10/21 (47.6%). At a 70.8‐month median follow‐up (FU), median RFS and median OS were not reached (NR) in patients with localized disease; median PFS and median OS were 6.3 (interquartile range [IQR]: 1.9–13.4) and 21.2 (IQR: 7.7–40.7) months in patients with advanced disease. Thirteen patients received MTX‐V (4 localized, 9 advanced disease), all evaluable for response. ORR was 7/13 (53.8%). At a 56.6‐month median FU, median RFS and median OS were 42.5 (IQR: 12.9–61.2) months and NR (no death events) in patients with localized disease, and NR (IQR: 24.9 to NR) and 83.4 months (IQR: 83.4 to NR) in patients with advanced disease. In the “other‐regimens group,” responses were seen in 3/4 patients treated with oral cyclophosphamide and 1/2 with docetaxel/gemcitabine.
Conclusion
Anthracycline‐based and MTX‐V regimens are very effective in IMT, with a similar ORR in both groups. MTX‐V achieved a prolonged disease control. Responses were also seen with oral cyclophosphamide and docetaxel/gemcitabine, but few patients were treated with these schedules.
Implications for Practice
Inflammatory myofibroblastic tumor (IMT) is an ultrarare sarcoma with known sensitivity to anaplastic lymphoma kinase (ALK) inhibitors in ALK‐fused cases, although ALK inhibitors are not licensed in the disease. The current knowledge on the activity of cytotoxic chemotherapy is limited. This multi‐institutional retrospective study on pediatric and adult patients with IMT shows that cytotoxic chemotherapy, and in particular anthracycline‐based and methotrexate plus/minus vinorelbine/vinblastine regimens, represents a treatment option and can be considered in IMT patients irrespectively from ALK status. This study provides a benchmark for future studies on new medical therapies.
Keywords: Sarcoma, Inflammatory myofibroblastic tumor, Chemotherapy, Doxorubicin, Methotrexate, Vinorelbine, Vinblastine
Short abstract
This article reports on the activity of cytotoxic chemotherapy in patients with inflammatory myofibroblastic tumors, focusing on anthracycline‐based regimens, the front‐line standard treatment in sarcomas, and methotrexate and/or vinorelbine or vinblastine. Data available on other cytotoxic regimens are also reported.
Introduction
Inflammatory myofibroblastic tumor (IMT) is an ultrarare soft tissue neoplasm, mainly diagnosed in children and young adults, with a slight female predominance, potentially arising at any site of the body [1, 2, 3, 4, 5, 6, 7, 8].
The 2013 World Health Organization (WHO) classification of soft tissue and bone sarcomas [9] defined an IMT as a neoplasm with intermediate malignant potential, rarely metastasizing [2, 10, 11], composed by myofibroblastic and fibroblastic spindle cells associated with an inflammatory infiltrate. Moreoften IMT harbors a fusion involving the anaplastic lymphoma kinase (ALK) gene, occurring in >50% of cases [12, 13, 14, 15]. ROS1, RET, and NTRK3 fusions have also been reported [16, 17, 18, 19, 20].
The natural history of IMTs is characterized by an indolent clinical behavior and a low tendency toward metastatic spread (<5% of cases) [2, 9, 10, 11]. A higher rate of distant metastases (about 5%–10%) has been reported for ALK‐negative IMTs [13, 21, 22]. Surgery is the standard of treatment for local disease, with a high chance of cure in completely resected cases. For patients with advanced disease, ALK inhibitors, such as crizotinib, can be active [23, 24]. Currently there are limited data regarding the role of conventional chemotherapy in this disease, and they are limited to small retrospective experiences, mainly focused on the pediatric population [25, 26, 27, 28, 29].
On this basis, the aim of this study was to retrospectively investigate the activity of cytotoxic chemotherapy in patients of any age with IMTs treated at nine European sarcoma reference centers, between 1996 and 2018. We focused on anthracycline‐based regimens, the front‐line standard treatment in sarcomas, and methotrexate and/or vinorelbine or vinblastine. Data available on other cytotoxic regimens were also collected.
Materials and Methods
We retrospectively collected data from all patients of any age, with a histologically confirmed diagnosis of IMT, treated with anthracycline‐based regimens, MTX‐V (e.g., cases treated with methotrexate plus vinorelbine or vinblastine and cases treated with vinca alkaloid alone), and other cytotoxic regimens between 1996 and 2018 at 9 European sarcoma reference centers that searched in their databases after an invitation was sent to 22. Data were extracted from institutional clinical databases. Both patients with localized disease treated with curative intent and those with advanced disease (locally advanced not eligible for surgical resection or definitive radiotherapy, or metastatic) were included. Two patients included in this case series had also been included in a retrospective study recently published by the European pediatric Soft Tissue Sarcoma Study Group (EpSSG) [29].
A written informed consent to treatment was obtained. Approval of the retrospective study by the institutional review board of each participating institution was obtained.
Diagnosis was reviewed and confirmed by a local expert sarcoma pathologist, according to WHO classification, 4th edition [10]. ALK status was assessed by immunohistochemistry and/or fluorescence in situ hybridization (FISH) in all cases.
Response was assessed by the local radiologist by reviewing retrospectively radiologic scans according to RECIST v1.1 [30].
In the anthracycline‐based regimens group, chemotherapy was administered every 3 weeks, corresponding to one cycle. In the MTX‐V group, chemotherapy with methotrexate and/or vinorelbine or vinblastine was administered weekly, and one cycle was defined as 4 weeks.
Statistical Analysis
Descriptive statistics and frequency tabulation were used to summarize patient and tumor characteristics.
The association between outcome and ALK status (positive vs. negative) was evaluated.
The primary endpoint of this study was the overall response rate (ORR) by RECIST v1.1; secondary endpoints were relapse‐free survival (RFS), for the patients treated for localized disease with curative intent, progression‐free survival (PFS), for the patients with advanced disease, and overall survival (OS). ORR was defined as the proportion of patients who achieved a complete response (CR) or a partial response (PR) according to RECIST v1.1 [30]. The corresponding 95% confidence intervals (CIs) were calculated based on the binomial distribution.
RFS, PFS, and OS were estimated using the Kaplan‐Meier method. Survival curves according to ALK status were compared using the log‐rank test.
For patients who were treated for localized disease with curative intent, RFS was calculated as the interval from day 1, cycle 1 of each treatment line to the date of the first evidence of recurrence, death, or the last follow‐up; for patients with advanced disease, PFS was calculated as the interval from the day 1, cycle 1 of each treatment line to the date of progressive disease (PD), death, or the last follow‐up; OS was calculated as the interval from day 1, cycle 1 of each treatment line to the time of death or the last follow‐up.
A two‐sided p < .05 was considered statistically significant. Statistical analyses were carried out with SAS (version 9.4; SAS Institute, Cary, NC) and R software (version 3.6.1).
Results
Thirty‐eight patients were retrospectively identified; 34 were evaluable for response, whereas 4 patients were treated in an adjuvant setting and were not assessable for response. All 38 patients were included in the survival analysis.
Table 1 summarizes population characteristics.
Table 1.
Main patient and disease characteristics
| Characteristics | Anthracycline‐based | MTX ± VNR/VNB | Other regimens | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Total | 25 | 13 | 10 | |||
| Age, median (IQR), years | 31 (22–46) | 16 (12–31) | 38 (30–61) | |||
| Gender | ||||||
| Male | 15 | 60.0 | 5 | 38.5 | 6 | 60.0 |
| Female | 10 | 40.0 | 8 | 61.5 | 4 | 40.0 |
| Primary site | ||||||
| Abdomen | 10 | 40.0 | 3 | 23.1 | 4 | 40.0 |
| Lung/thoracic wall | 8 | 32.0 | 3 | 23.1 | 3 | 30.0 |
| Other | 7 | 28.0 | 7 | 53.8 | 3 | 30.0 |
| ALK status | ||||||
| Positive | 18 | 72.0 | 6 | 46.2 | 7 | 70.0 |
| IHC | 2 | 11.1 | 3 | 50.0 | 2 | 28.6 |
| FISH | 7 | 38.9 | 3 | 50.0 | 1 | 14.3 |
| Both | 9 | 50.0 | 0 | ‐ | 4 | 57.1 |
| Negative | 7 | 28.0 | 7 | 53.8 | 3 | 30.0 |
| IHC | 5 | 71.4 | 4 | 57.1 | 1 | 33.3 |
| FISH | — | — | — | — | — | — |
| Both | 2 | 28.6 | 3 | 42.9 | 2 | 66.7 |
| Stage at treatment start | ||||||
| Local | 8 | 32.0 | 4 | 30.8 | — | — |
| Locally advanced | 3 | 12.0 | 5 | 38.5 | 6 | 60.0 |
| Metastatic | 14 | 56.0 | 4 | 30.8 | 4 | 40.0 |
| Chemotherapy regimen | ||||||
| A | 8 | 32.0 | ||||
| AI | 14 | 56.0 | ||||
| A + other | 3 | 12.0 | ||||
| MTX + VNB | 9 | 69.2 | ||||
| MTX + VNR | 3 | 23.1 | ||||
| VNR/VNB | 1 | 7.7 | ||||
| CTX | 4 | 40.0 | ||||
| T | 2 | 20.0 | ||||
| GD | 2 | 20.0 | ||||
| CBDCA + TXL | 1 | 10.0 | ||||
| VP‐16 | 1 | 10.0 | ||||
| Number of prior lines, median (range) | 0 (0–3) | 0 (0–2) | 1 (0–3) | |||
| Treatment line | ||||||
| First line | 21 | 84.0 | 10 | 76.9 | 3 | 30.0 |
| Second line | 2 | 8.0 | 2 | 15.4 | 4 | 40.0 |
| Further line | 2 | 8.0 | 1 | 7.7 | 3 | 30.0 |
Abbreviations: A, adriamycin; AI, adriamycin and ifosfamide; ALK, anaplastic lymphoma kinase; CBDCA + TXL, carboplatin and paclitaxel; CTX, oral cyclophosphamide; FISH, fluorescence in situ hybridization; GD, gemcitabine and docetaxel; IHC, immunohistochemistry; IQR, interquartile range; MTX, methotrexate; T, trabectedin; VNB, vinblastine; VNR, vinorelbine; VNR/VNB, vinorelbine or vinblastine alone; VP‐16, oral etoposide.
Of 38 patients, 24 (63.2%) had ALK‐positive and 14 (36.8%) had ALK‐negative disease (Table 1). Twenty‐five of 38 (65.8%) patients were treated with anthracycline‐based chemotherapy, 13/38 (34.2%) with MTX‐V chemotherapy, and 10/38 (26.3%) with other regimens (in detail: 1 carboplatin plus paclitaxel, 1 oral etoposide, 2 docetaxel plus gemcitabine, 2 trabectedin, 4 oral cyclophosphamide).
Overall, the median number of received treatment lines was 2 (range 1–8). Six out of 25 (24.0%) patients in the anthracycline‐based group and 2/13 (15.4%) patients in the MTX‐V group received an ALK inhibitor after chemotherapy. The median follow‐up time for the entire series was 69 months (interquartile range [IQR]: 39.3–139.5).
In the anthracycline‐based group, 8/25 (32.0%) patients were treated for localized disease with curative intent and 17/25 (68.0%) for advanced disease. Eighteen out of 25 (72.0%) patients had ALK‐positive and 7/25 (28.0%) patients had ALK‐negative disease. Twenty‐one out of 25 (84.0%) patients were evaluable for response, whereas 4/25 (16.0%) were nonevaluable for response because they were treated in an adjuvant setting. The median number of cycles was 6 (range 1–6). Twenty‐one out of 25 (84.0%) patients received anthracyclines as first‐line treatment, 2/25 (8.0%) as second‐line treatment, and 2/25 (8.0%) in further line. Eight out of 25 (32.0%) patients received an anthracycline as single agent, 14/25 (56.0%) in combination with ifosfamide, and 3/25 (12.0%) in combination with other drugs (e.g., cisplatin, dacarbazine, vincristine, or etoposide). All patients in this group had completed anthracycline‐based treatment at the time of this analysis: 13/25 (52.0%) for having received the maximum cumulative dose of anthracycline, 7/25 (28.0%) for PD, 2/25 (8.0%) for grade 3 hematological toxicity, and 3/25 (12.0%) for surgery after two, three, and four cycles of chemotherapy, respectively.
Grade (G) 3 or G4 adverse events were observed in 7/25 (28%) patients of this group: G4 febrile neutropenia in 2 patients, G3 neutropenia in 5, G3 anemia in 2, and G4 thrombocytopenia in 2.
Best RECIST response was 10/21 PR (47.6%), 6/21 stable disease (SD) (28.6%), and 5/21 PD (23.8%), for an ORR of 47.6% (95% CI: 25.7%–70.2%). Responses were observed both in ALK‐positive (8/16 evaluable patients, 50.0%) and in ALK‐negative (2/5 evaluable patients, 40.0%) cases.
For patients with localized disease (8 cases, 4 not evaluable since treated in adjuvant setting), best RECIST response was 2/4 PR (50.0%) and 2/4 SD (50.0%), for an ORR of 50.0% (95% CI: 6.8%–93.2%), At a median follow‐up of 70.8 months (IQR: 39.3–89.3), median RFS and median OS were not reached; 5‐year RFS and 5‐year OS rates were 50.0% (95% CI: 25.0%–100.0%) and 75.0% (95% CI: 50.3%–100.0%), respectively.
For patients with advanced disease (17 cases), best RECIST response was 8/17 PR (47.1%), 4/17 SD (23.5%), and 5 PD (29.4%), for an ORR of 47.1% (95% CI: 23.0%–72.2%). Median PFS and OS were 6.3 (IQR: 1.9–13.4) and 21.2 (IQR: 7.7–40.7) months, respectively; the 1‐year PFS rate was 29.4% (95% CI: 14.1%–61.4%), whereas the 2‐year OS rate was 41.2% (95% CI: 23.3%–72.7%; Fig. 1). Median OS was 22.4 months (IQR: 3.2–40.7) in ALK‐positive and 17.3 months (IQR: 10.5–25.7) in ALK‐negative advanced cases (p = .351). Median PFS was 4.7 months (IQR: 1.3–17.0) in ALK‐positive and 8.5 months (IQR: 5.4–12.1) in ALK‐negative advanced cases (p = .875). No association was found between RFS, PFS, OS, and ALK status in this treatment group.
Figure 1.
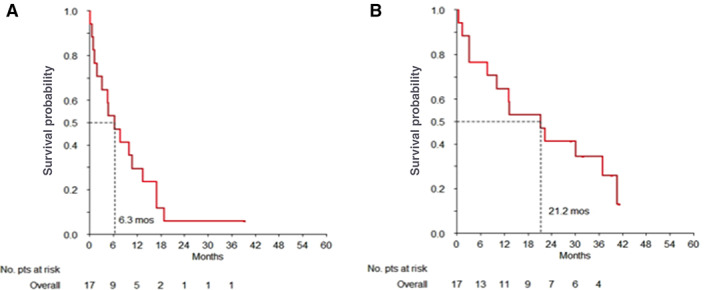
Overall survival curves of inflammatory myofibroblastic tumor patients treated with anthracycline‐based chemotherapy. (A) Kaplan‐Meier curve of progression free survival and (B) of overall survival.
In the MTX‐V group, 4/13 patients (30.8%) were treated for local disease and 9/13 patients (69.2%) for advanced disease. Six out of 13 (46.2%) patients had ALK‐positive and 7/13 (53.8%) ALK‐negative disease. All patients in this group were evaluable for response. The median number of cycles was 38 (range 2–50). Ten out of 13 (76.9%) patients received MTX‐V as first‐line treatment, 2/13 (15.4%) as second‐line treatment, and 1/13 as further line (7.7%). Nine out of 13 (69.2%) patients received methotrexate in combination with vinblastine, 3/13 (23.1%) in combination with vinorelbine, and 1/13 (7.7%) received vinorelbine as monotherapy. All patients of this group had completed their treatment at the time of this analysis: 6/13 (46.2%) for clinical decision after prolonged disease control (1 patient after 6 months, 3 patients after 10 months, and 2 patients after 1 year from treatment start), 4/13 (30.8%) for PD, and 3/13 (23.1%) to undergo surgery.
G3 or G4 toxicity events occurred in 2 (15%) of 13 patients in this group: G3 hypertransaminasemia in 1 patient and G3 mucositis in 1 case. Two patients achieved a CR and were still disease free at the time of the analysis, after 25 and 60 months from treatment start. Best RECIST response was 2/13 CR (15.4%), 5/13 PR (38.5%), 3/13 SD (23.1%), and 3/13 PD (23.1%), for an ORR of 53.8% (95% CI: 25.1%–80.8%; Fig. 2).
Figure 2.
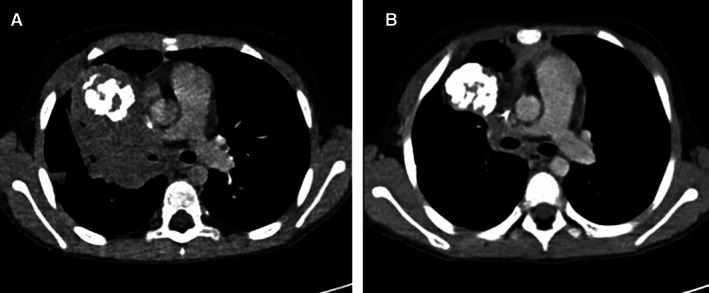
Response according to RECIST to methotrexate plus vinorelbine in a patient with a metastatic para‐mediastinal inflammatory myofibroblastic tumor (IMT). (A): Contrast‐enhanced computed tomography scan image on an axial plane shows a right para‐mediastinal IMT involving hilum at baseline. (B): After 10 cycles of treatment with MTX‐V, there was evidence of a 37% decrease in tumor size, corresponding to a partial response by RECIST.
Responses were observed both in ALK‐positive (4/6, 66.7%) and in ALK‐negative (3/7, 42.9%) disease.
For patients with localized disease (4 cases), best RECIST response was 2/4 PR (50.0%), 1/4 SD (25.0%), and 1 PD (25.0%), for an ORR of 50.0% (95% CI: 6.8%–93.2%). At a median follow‐up time of 56.6 months (IQR: 27.8–129.9), median RFS was 42.5 (IQR: 12.9–61.2) months, whereas median OS was not estimated as there were no death events in this subgroup.
For patients with advanced disease (9 cases) best RECIST response was 2/9 CR (22.2%), 3/9 PR (33.3%), 2/9 SD (22.2%), and 2/9 PD (22.2%), for an ORR of 55.6% (95% CI: 21.2%–86.3%). Median PFS was not reached (IQR: 24.9 to NR); 1‐/2‐year and 5‐year PFS rates were 77.8% (95% CI: 54.9%–100.0%) and 62.2% (95% CI: 35.5%–100.0%), respectively; median OS was 83.4 months (IQR: 83.4 to NR; Fig. 3). Median PFS was not reached in ALK‐positive and ALK‐negative advanced cases. No association was found between RFS, PFS, OS, and ALK status in this treatment group.
Figure 3.
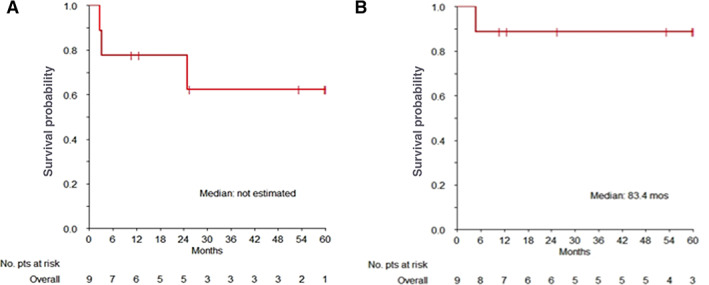
Overall survival curves of inflammatory myofibroblastic tumor patients treated with methotrexate and vinorelbine/vinblastine chemotherapy. (A) Kaplan‐Meier curve of progression free survival and (B) of overall survival.
In the other‐regimens group, all patients were treated for advanced disease. Seven out of 10 (70%) patients had ALK‐positive and 3/10 (30%) ALK‐negative disease. All patients were evaluable for response. Three out of 10 patients received chemotherapy as first‐line treatment, 4/10 as second‐line treatment, and 3/10 as further line. Four out of 10 patients received oral cyclophosphamide, 2/10 docetaxel plus gemcitabine, 2/10 trabectedin, 1/10 carboplatin plus paclitaxel, and 1/10 oral etoposide.
Responses were seen with oral cyclophosphamide (one CR and two PR out of four patients) and docetaxel plus gemcitabine (one PR out of two patients). Responses to both regimens were observed in ALK‐positive cases. No association was found between RFS, PFS, OS, and ALK status in this treatment group.
Figure 4 shows the progression‐free survival by RECIST in each treatment group.
Figure 4.
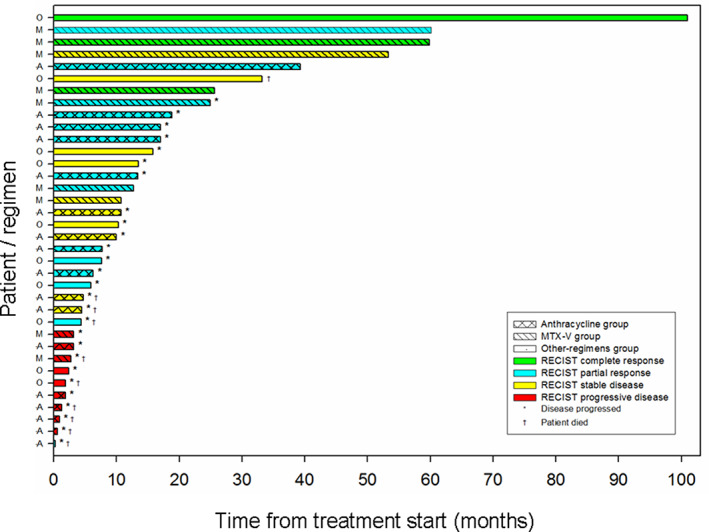
Swimmer‐plot showing the progression‐free survival by RECIST in each treatment group. Abbreviations: A, anthracycline‐based chemotherapy group (coarse‐hatched bar pattern); M, MTX‐V group (single, oblique‐hatched bar pattern); O, other chemotherapeutic regimens group (solid bar pattern).
Discussion
This academic, multi‐institutional, European retrospective study collected the largest series currently available of patients affected by IMTs treated with cytotoxic chemotherapy, confirming that cytotoxic agents have a high degree of activity in the disease, superior to what is expected in adults with soft tissue sarcoma treated with the same agents. In particular, anthracycline‐based regimens were associated with an ORR of 48%, and a median PFS of 6.3 months in patients with advanced disease. The ORR with MTX‐V was 54%, with a prolonged control of disease (median PFS not reached at a median follow‐up of 57 months). Responses were observed with oral cyclophosphamide and gemcitabine plus docetaxel, although few patients were treated with these regimens.
IMT is an ultrarare sarcoma, mostly diagnosed in young patients and often arising from critical anatomic sites, such as the pulmonary hilum. Although most IMTs at diagnosis are localized and can be cured with surgery, systemic therapies still play a crucial role for patients with locally advanced disease (particularly in challenging primary sites) and in those developing metastatic lesions. Because of the extreme rarity of IMTs, data on chemotherapy in this sarcoma subtype are limited, retrospective, and mostly focused on childhood [25, 26, 27, 28, 29].
The results of this study are limited by its small sample size and its retrospective nature, which implies a possible degree of variability in patients’ follow‐up and imaging assessment, and does not allow to perform additional subgroup analyses to investigate the differential activity of the investigate regimens in different patient populations. However, dedicated prospective studies on conventional chemotherapy are unlikely to happen in this histology. Indeed, this is the largest retrospective series available. We have included not only children but patients of any age. In this study, pathologic diagnosis was confirmed in all cases by an expert sarcoma pathologist and ALK was systematically assessed by immunohistochemistry and/or FISH. ALK‐positive cases were 63%, in line with what is reported in the literature, even though we could not sequence ALK‐negative cases to definitively rule out the presence of an ALK fusion with uncommon breakpoints or other translocation involving ROS, RET, or NTRK in these cases. This could have had underestimated the proportion of ALK‐positive patients in the series [17, 18, 19, 20].
This study confirms the activity of anthracycline‐based regimens, which remains the front‐line standard treatment in sarcomas including advanced IMTs, and of MTX‐V in a high proportion of patients (ORR: 48% and 54%, respectively). Responses were observed irrespectively to ALK status. Our results are consistent with what has previously been reported [25, 26, 27, 28, 29].
Kube et al. described the outcome of 11 patients <21 years of age with IMTs treated with regimens usually administered in children with rhabdomyosarcoma, including dactinomycin, ifosfamide (or cyclophosphamide), and vincristine, with or without doxorubicin [28], with 3 responses. Kube also reported two additional cases treated with methotrexate plus vinorelbine, dexamethasone, and ibuprofen with one response. Recently, Casanova et al. reported on the retrospective experience of the EpSSG. Two cases in this series were also included in our study. A total of 8 objective responses were seen in 10 patients aged 0–24 years with IMTs treated with vinblastine and methotrexate, whereas no responses were identified in 3 patients treated with ifosfamide‐based chemotherapy [29]. Interestingly, our series, which confirmed the impressive activity of the MTX‐V regimen on a similar number of patients, also indicates prolonged disease control with this schedule, in excess of 2 years, similarly to that observed in another mesenchymal tumor, desmoid fibromatosis [31].
Finally, we report the first response of about 6 months to gemcitabine and docetaxel in an ALK‐positive advanced IMT refractory to anthracycline‐based chemotherapy.
To note, anecdotal reports of response to nonsteroidal anti‐inflammatory drugs and steroids are also available in IMT [32, 33, 34, 35], but we did not collect data on those approaches in this disease.
Prospective data available in the literature on systemic treatment of IMTs come from the European Organization for Research and Treatment of Cancer 90101‐CREATE phase II study, which investigated the activity of the ALK inhibitor crizotinib in 19 patients with advanced IMT, observing 6 RECIST responses of 12 ALK‐positive and 1 of 7 ALK‐negative patients. Median duration of response was 9.0 months in ALK‐positive and 7.6 months in ALK‐negative disease, with a 12‐month PFS rate of 67% [36]. Similar results were observed in a phase II study on pediatric patients with ALK‐positive advanced IMT, which detected 12 RECIST responses out of 14 patients [37]. Both studies also showed that crizotinib was very well tolerated, thus supporting the use of ALK inhibitors in patients with ALK‐positive IMTs. Unfortunately, neither crizotinib nor other ALK inhibitors are formally approved to treat this tumor, and in addition, their activity is limited in ALK‐negative patients. It is imperative that this class of agent is approved for IMT; however, cytotoxic chemotherapy represents an alternative treatment option and can also be considered in patients with IMTs not harboring an ALK translocation. Our study does not allow any comparison between the activity and toxicity profile of cytotoxic chemotherapy to ALK inhibitors, although the 1‐year PFS rate with MTX‐V compares well with that observed with targeted agents. This should be confirmed in a comparative prospective fashion, which is difficult to foresee considering the rarity of the disease. Our data show that both classes of agents may be used in a sequential approach in advanced disease requiring systemic therapy.
Conclusion
This retrospective analysis shows that international cooperative efforts involving both pediatric and adult oncologists are feasible in ultrarare sarcomas. This effort proves that anthracycline‐based and MTX‐V regimens have a high degree of activity in IMT, with a similar ORR in both groups and prolonged disease control observed with MTX‐V. Anecdotal responses were seen with oral cyclophosphamide and docetaxel plus gemcitabine.
Author Contributions
Conception/design: Giacomo Giulio Baldi, Silvia Stacchiotti
Provision of study material or patients: Mehdi Brahmi, Elena Cojocaru, Olivier Mir, Michela Casanova, Bruno Vincenzi, Tommaso Martino De Pas, Giovanni Grignani, Maria Abbondanza Pantaleo, Jean Yves Blay, Robin Lewis Jones, Axel Le Cesne, Alessandro Gronchi, Paola Collini, Angelo Paolo Dei Tos, Carlo Morosi, Silvia Stacchiotti
Collection and/or assembly of data: Giacomo Giulio Baldi, Mehdi Brahmi, Elena Cojocaru, Olivier Mir, Michela Casanova, Bruno Vincenzi, Tommaso Martino De Pas, Giovanni Grignani, Maria Abbondanza Pantaleo
Data analysis and interpretation: Giacomo Giulio Baldi, Salvatore Lo Vullo, Luigi Mariani, Silvia Stacchiotti
Manuscript writing: Giacomo Giulio Baldi, Salvatore Lo Vullo, Anna Maria Frezza, Paolo Giovanni Casali, Silvia Stacchiotti
Final approval of manuscript: Giacomo Giulio Baldi, Mehdi Brahmi, Salvatore Lo Vullo, Elena Cojocaru, Olivier Mir, Michela Casanova, Bruno Vincenzi, Tommaso Martino De Pas, Giovanni Grignani, Maria Abbondanza Pantaleo, Jean Yves Blay, Robin Lewis Jones, Axel Le Cesne, Anna Maria Frezza, Alessandro Gronchi, Paola Collini, Angelo Paolo Dei Tos, Carlo Morosi, Luigi Mariani, Paolo Giovanni Casali, Silvia Stacchiotti
Disclosures
Giacomo G. Baldi: Eli Lilly & Co., Eisai, PharmaMar (H); PharmaMar, Pfizer, Eli Lilly & Co. (other: travel grants); AboutEvents, EditaMed, Eli Lilly & Co. (SAB); Olivier Mir: Amgen, AstraZeneca, Bayer, Bristol‐Myers Squibb, Eli Lilly and Company, Ipsen, Lundbeck, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Servier, Vifor Pharma (C/A), Amplitude Surgical, Ipsen, Transgene (OI); Bruno Vincenzi: Eisai, Eli Lilly & Co., Novartis, PharmaMar, Abbot (H), Eli Lilly & Co., Novartis, PharmaMar (RF); Eli Lilly & Co., Novartis, PharmaMar (RF‐Institutional); Giovanni Grignani: Pharmamar, Bayer, Eli Lilly and Company, Eisai, Novartis (SAB), Pfizer, Tesaro (other); Jean Yves Blay: Roche, Bayer, Pfizer (H, RF); Robin Lewis Jones: Merck Sharp & Dohme, GlaxoSmithKline (RF), Adaptimmune, Athenex, Blueprint Medicines, Clinigen, Eisai, Epizyme, Daichii, Deciphera, Immunedesign, Eli Lilly and Company, Merck, Pharmamar, UpToDate (C/A); Axel Le Cesne: Bayer, Deciphera, Blueprint (H); Anna Maria Frezza: Amgen Dompé, AROG Bayer, Blueprint Medicines, Eli Lilly & Co., Daiichi Sankyo Pharma, Epizyme, GlaxoSmithKline, Novartis, Pfizer, PharmaMar, Advenchen, Karyopharm (RF: Institutional); PharmaMar (Other: travel grants); Angelo P. Dei Tos: Bayer, Roche (C/A); PharmaMar (Other: travel grants); Paolo Giovanni Casali: Bayer, Deciphera, Eisai, Eli Lilly and Company, Nektar Therapeutics, Pfizer (H, C/A), Advenchen Laboratories, Amgen Dompé, AROG Pharmaceuticals, Bayer, Blueprint Medicines, Daiichi Sankyo, Deciphera, Eisai, Eli Lilly and Company, Epizyme Inc, GlaxoSmithKline, Karyopharm Pharmaceuticals, Novartis, Pfizer, Pharmamar (RF); Silvia Stacchiotti: Bayer, Bavarian Nordic, Deciphera, Daiichi, Eli Lilly & Co., Epizyme, Karyopharm, MaxiVax, Pharmamar, Takeda (C/A), Eli Lilly & Co., Pharmamar (H); Pharmamar (Other: travel grants); Advenchen, Amgen Dompé, AROG, Bayer, Blueprint Medicines, Daiichi Sankyo Pharma, Deciphera, Eli Lilly & Co., Epizyme, GlaxoSmithKline, Karyopharm, Novartis, Pfizer, PharmaMar, SpringWorks (RF: Institutional). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Meis JM, Enzinger FM. Inflammatory fibrosarcoma of the mesentery and retroperitoneum. A tumor closely simulating inflammatory pseudotumor. Am J Surg Pathol 1991;15:1146–1156. [DOI] [PubMed] [Google Scholar]
- 2. Coffin CM, Watterson J, Priest JR et al. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol 1995;19:859–872. [DOI] [PubMed] [Google Scholar]
- 3. Myint MA, Medeiros LJ, Sulaiman RA et al. Inflammatory pseudotumor of the ileum. A report of a multifocal, transmural lesion with regional lymph node involvement. Arch Pathol Lab Med 1994;118:1138–1142. [PubMed] [Google Scholar]
- 4. Wenig BM, Devaney K, Bisceglia M. Inflammatory myofibroblastic tumor of the larynx. A clinicopathologic study of eight cases simulating a malignant spindle cell neoplasm. Cancer 1995;76:2217–2229. [DOI] [PubMed] [Google Scholar]
- 5. Sciot R, Dal Cin P, Fletcher CD et al. Inflammatory myofibroblastic tumor of bone: Report of two cases with evidence of clonal chromosomal changes. Am J Surg Pathol 1997;21:1166–1172. [DOI] [PubMed] [Google Scholar]
- 6. Ramachandra S, Hollowood K, Bisceglia M et al. Inflammatory pseudotumour of soft tissues: A clinicopathological and immunohistochemical analysis of 18 cases. Histopathology 1995;27:313–323. [DOI] [PubMed] [Google Scholar]
- 7. Hausler M, Schaade L, Ramaekers VT et al. Inflammatory pseudotumors of the central nervous system: Report of 3 cases and a literature review. Hum Pathol 2003;34:253–262. [DOI] [PubMed] [Google Scholar]
- 8. Rabban JT, Zaloudek CJ, Shekitka KM et al. Inflammatory myofibroblastic tumor of the uterus: A clinicopathologic study of 6 cases emphasizing distinction from aggressive mesenchymal tumors. Am J Surg Pathol 2005;29:1348–1355. [DOI] [PubMed] [Google Scholar]
- 9. Coffin CM, Fletcher JA. Inflammatory myofibroblastic tumour In: Fletcher CDM, Bridge JA, Hongendoorn PCW. et al., eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed, Lyon, France: International Agency for Research on Cancer, 2013:83–84. [Google Scholar]
- 10. Janik JS, Janik JP, Lovell MA et al. Recurrent inflammatory pseudotumors in children. J Pediatr Surg 2003;38:1491–1495. [DOI] [PubMed] [Google Scholar]
- 11. Cerfolio RJ, Allen MS, Nascimento AG et al. Inflammatory pseudotumors of the lung. Ann Thorac Surg 1999;67:933–936. [DOI] [PubMed] [Google Scholar]
- 12. Cook JR, Dehner LP, Collins MH et al. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumour: A comparative immunohistochemical study. Am J Surg Pathol 2001;25:1364–1371. [DOI] [PubMed] [Google Scholar]
- 13. Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumour: Comparison of clinico‐pathologic, hostologic and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 2007;31:509–520. [DOI] [PubMed] [Google Scholar]
- 14. Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: Where are we now? J Clin Pathol 2008;61:428–437. [DOI] [PubMed] [Google Scholar]
- 15. Mossé YP, Voss SD, Lim MS et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: A Children's Oncology Group Study. J Clin Oncol 2017;35:3215–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lovly CM, Gupta A, Lipson D et al. Inflammatory myofibroblastic tumour harbor multiple potentially actionable kinase fusions. Cancer Discov 2014;4:889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Antonescu CR, Suurmeijer AJ, Zhang L et al. Molecular characterization of inflammatory myofibroblastic tumours with frequent ALK and ROS1 gene fusions and rare novel RET rearrangements. Am J Surg Pathol 2015;39:957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamamoto H, Yoshida A, Taguchi K et al. ALK, ROS1 and NTRK3 gene rearrangements in inflammatory myofibroblastic tumours. Histopathology 2016; 69:72–83. [DOI] [PubMed] [Google Scholar]
- 19. Lopez‐Nunez O, John I, Panasiti R et al. Infantile inflammatory myofibroblastic tumors: Clinicopathological and molecular characterization of 12 cases. Mod Pathol 2020;33:576–590. [DOI] [PubMed] [Google Scholar]
- 20. Chang JC, Zhang L, Drilon AE et al. Expanding the molecular characterization of thoracic inflammatory myofibroblastic tumours beyond ALK gene rearrangements. J Thorac Oncol 2019;14:825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chun YS, Wang L, Nascimento AG et al. Pediatric inflammatory myofibroblastic tumor: Anaplastic lymphoma kinase (ALK) expression and prognosis. Pediatr Blood Cancer 2005;45:796–801. [DOI] [PubMed] [Google Scholar]
- 22. Marino‐Enriquez A, Wang WL, Roy A et al. Epithelioid inflammatory myofibroblastic sarcoma: An aggressive intra‐abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol 2011;35:135–144. [DOI] [PubMed] [Google Scholar]
- 23. Webb TR, Slavish J, George RE et al. Anaplastic lymphoma kinase: Role in cancer pathogenesis and small‐molecule inhibitor development for therapy. Expert Rev Anticancer Ther 2009;9:331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blackhall F, Cappuzzo F. Crizotinib: From discovery to accelerated development to front‐line treatment. Ann Oncol 2016;27(suppl 3):iii35–iii41. [DOI] [PubMed] [Google Scholar]
- 25. Dalton BG, Thomas PG, Sharp N et al. Inflammatory myofibroblastic tumors in children. J Pediatr Surg 2016;51:541–544. [DOI] [PubMed] [Google Scholar]
- 26. Alaggio R, Cecchetto G, Bisogno G et al. Inflammatory myofibroblastic tumours in childhood: A report from the Italian Cooperative Group studies. Cancer 2010;116:216–226. [DOI] [PubMed] [Google Scholar]
- 27. Favini F, Resti AG, Collini P et al. Inflammatory myofibroblastic tumour of the conjunctiva: Response to chemotherapy with low‐dose methotrexate and vinorelbine. Pediatr Blood Cancer 2010;54:483–485. [DOI] [PubMed] [Google Scholar]
- 28. Kube S, Vokuhl C, Dantonello T et al. Inflammatory myofibroblastic tumours – A retrospective analysis of the Cooperative Weichteilsarkom Studien‐Gruppe. Pediatr Blood Cancer 2018;65:e27012. [DOI] [PubMed] [Google Scholar]
- 29. Casanova M, Brennan B, Alaggio R et al. Inflammatory myofibroblastic tumour: Experience of European pediatric Soft Tissue Sarcoma Study Group (EpSSG). Eur J Cancer 2020;127:123–129. [DOI] [PubMed] [Google Scholar]
- 30. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 31. Palassini E, Frezza AM, Mariani L et al. Long term efficacy of methotrexate and vinblastine/vinorelbine in a large series of patients affected by desmoid‐type fibromatosis. Cancer J 2017;23:86–91. [DOI] [PubMed] [Google Scholar]
- 32. Su W, Ko A, O'Connell T et al. Treatment of pseudotumours with nonsteroidal antinflammatory drugs. J Pediatr Surg 2000;35:1635–1637. [DOI] [PubMed] [Google Scholar]
- 33. Berger A, Kim C, Hagstrom N et al. Successful preoperative treatment of pediatric bladder inflammatory myofibroblastic tumour with antinflammatory therapy. Urology 2007;70:372.e13–e15. [DOI] [PubMed] [Google Scholar]
- 34. Chavez C, Hoffman MA. Complete remission of ALK‐negative plasma cell granuloma (inflammatory myofibroblastic tumour) of the lung induced by celecoxib: A case report and review of literature. Oncol Lett 2013;5:1672–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Applebaum H, Kieran MW, Cripe TP et al. The rationale for nonsteroidal anti‐inflammatory drug therapy for inflammatory myofibroblastic tumours: A Children's Oncology Group study. J Pediatr Surg 2005;40:999–1003. [DOI] [PubMed] [Google Scholar]
- 36. Shoffski P, Sufliarsky J, Gelderblom H et al. Crizotinib in patients with advanced, inoperable inflammatory myofibroblastic tumors with and without anaplastic lymphoma kinase gene alterations (European Organization for Research and Treatment of Cancer 90101 CREATE): A multicentre, single‐drug, prospective, non‐randomized phase 2 trial. Lancet Respir Med 2018;6:431–441. [DOI] [PubMed] [Google Scholar]
- 37. Mossé YP, Voss SD, Lim MS et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: A Children's Oncology Group Study. J Clin Oncol 2017;35:3215–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]


