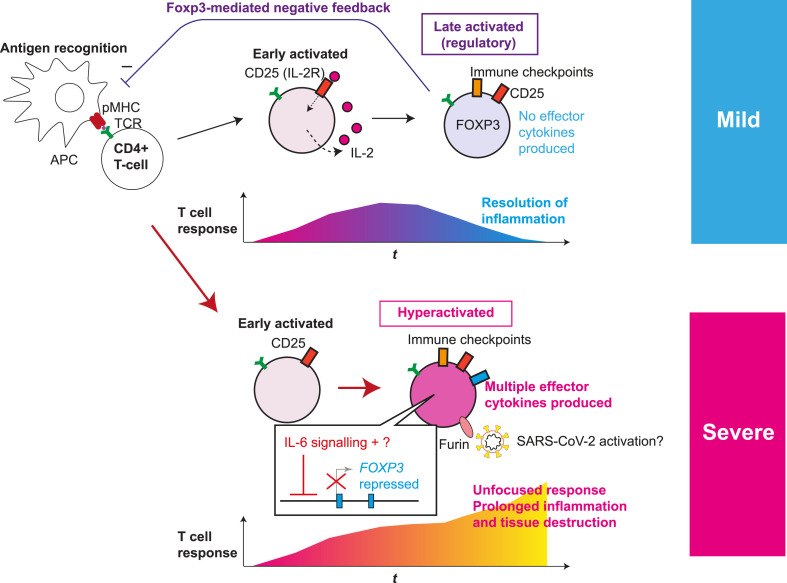
Fig. 1.
Roles of T-cell hyperactivation in the lung of severe COVID-19 patients. The fates of activated T-cells in mild and severe COVID-19 patients are depicted. Antigen-presenting cells (APC) present antigens as peptide-MHC complex (pMHC) to CD4+ T-cells, which triggers T-cell receptor (TCR) signalling and subsequent activation and differentiation processes. Initially, early activated T-cells start to produce CD25 and IL-2, establishing a positive feedback loop for T-cell activation and proliferation. Subsequently the fates of activated T-cells are different in mild and severe COVID-19 patients. (Upper panel) In normal conditions and mild patients, IL-2 signalling enhances FOXP3 transcription in a part of activated T-cells. In addition, the expression of immune checkpoint molecules such as CTLA-4 is increased. Meanwhile, some T-cells can differentiate into Tfh and Th cells (not shown). CD25+CTLA-4+FOXP3+ T-cells can consume and occupy immunological resources including IL-2 and CD28 signalling, and thereby suppress T-cell activation, leading to resolution of inflammation. (Lower panel) In severe COVID-19, activated T-cells fail to express FOXP3 while further enhancing the expression of CD25 and immune checkpoint molecules, producing multiple Th cytokines and showing the features of hyperactivated T-cells. FOXP3 repression is likely due to the increased IL-6 availability and undefined factors. In addition, CD25+ hyperactivated T-cells produce FURIN, which may further enhance the viral infection, promoting prolonged inflammation and tissue destruction. Figure modified from Ref. [33].