Figure 1.
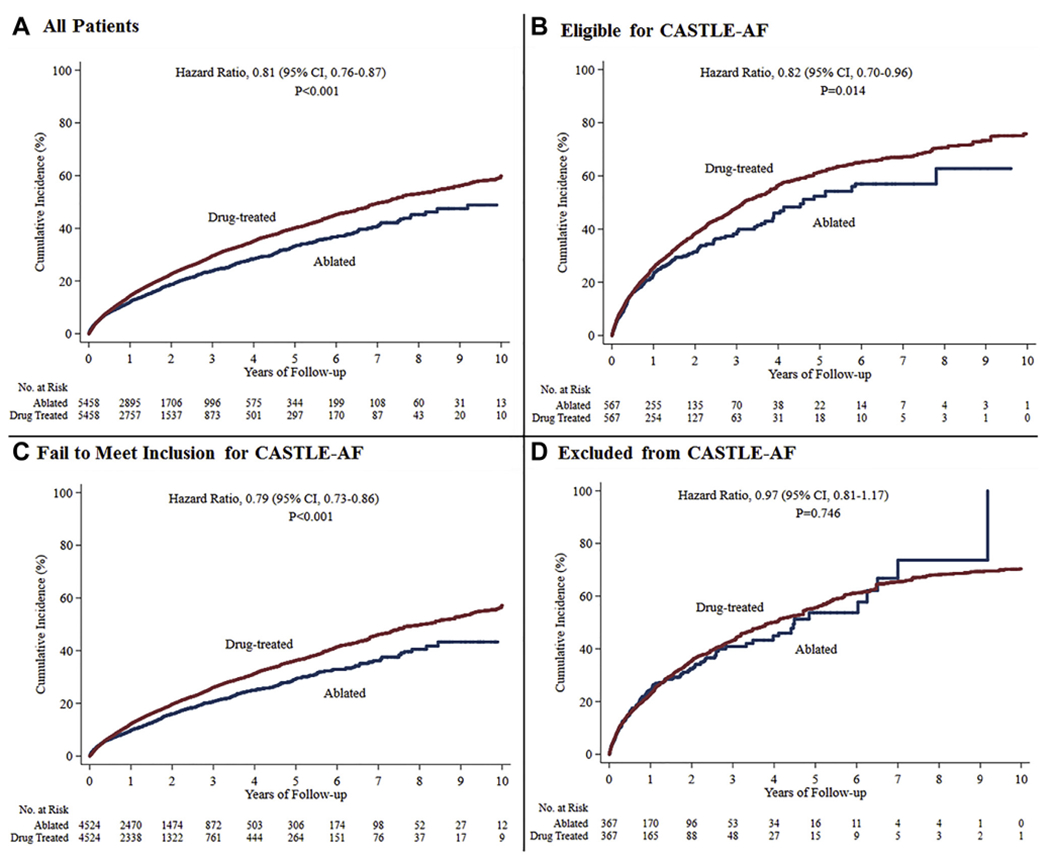
Primary end point (composite of all-cause mortality and heart failure hospitalization) in ablated or drug-treated patients with atrial fibrillation and heart failure, stratified by Catheter Ablation for Atrial Fibrillation with Heart Failure (CASTLE-AF) trial eligibility criteria. The cumulative incidence in the overall cohort (A), in patients who would be potentially eligible for CASTLE-AF (B), in patients who failed to meet the inclusion criteria (C), and in patients who met at least one of the trial exclusion criteria (D). Drug-treated patients are the reference group in Cox proportional hazards regression analyses. All the curves and numbers were generated using propensity score weighting. CI = confidence interval.
