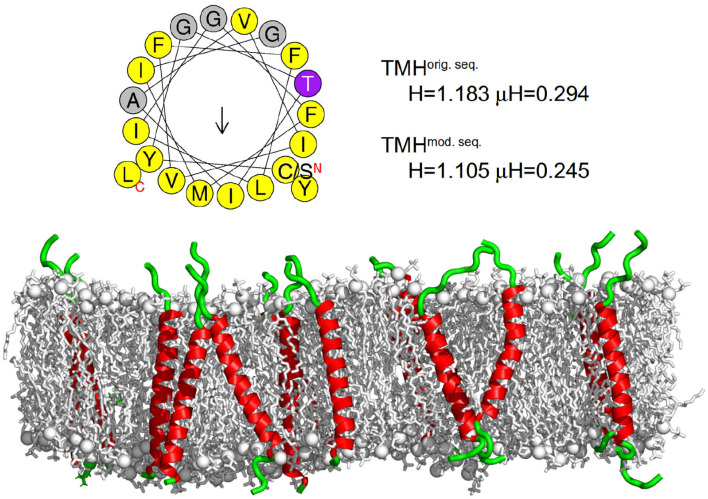
Figure 2.
Transferrin transmembrane sequence and structure. (Top) Wheel projection of the transmembrane helices (TMH) and the corresponding helicity (H) and hydrophobic moment (μH) for both sequences (please note the only substitution of the N terminal cysteine for serine) as determined by HELIQUEST (Gautier et al., 2008). (Bottom) Visualization of the secondary structure of the membrane embedded transferrin TMHs after 500 ns of the MD simulation. The membrane is represented as gray sticks with phosphates highlighted as spheres, the helical part of the peptide is shown in red and the unstructured part in green. The N termini of all peptides are at the bottom and the C termini at the top. On average, transferrin contained 24 helical residues.