Fig. 2.
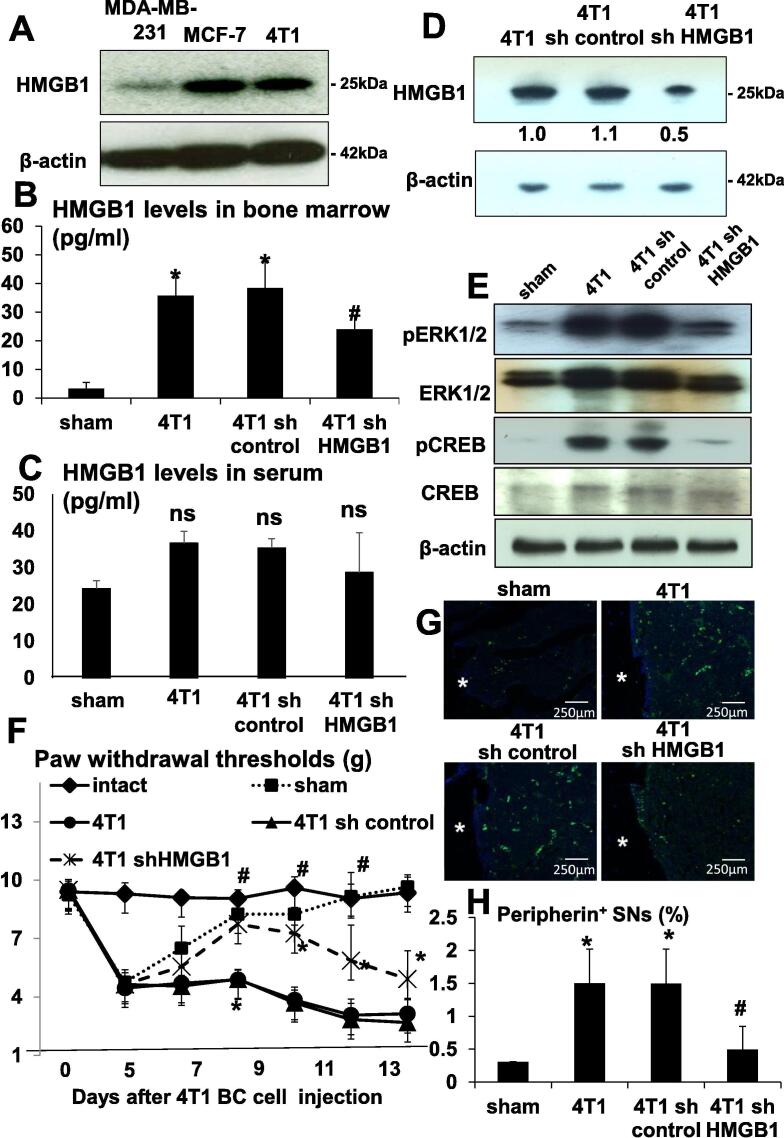
Role of HMGB1 produced by 4T1 BC in BCABP. (A) Expression of HMGB1 in mouse 4T1 BC and human BC MDA-MB-231 and MCF-7 cells in culture. Whole cell lysates were subjected to Western analysis. HMGB1 levels in the bone marrow of tibiae by ELISA. Bone marrow fluids were collected as described in Methods from sham, 4T1, 4T1/sh control and 4T1/sh HMGB1 mice. (B) HMGB1 levels were increased in the bone marrow of tibiae of 4T1 and 4T1/sh control mice, while significantly decreased in the bone marrow of tibiae of 4T1/sh HMGB1 mice. Data are shown as mean ± SD (n = 8). * Significantly different from sham mice (p < 0.05). # Significantly different from 4T1 and 4T1/sh control mice (p < 0.05). (C) HMGB1 levels in circulating serum determined by ELISA. Sera were collected as described in Methods from sham, 4T1, 4T1/sh control and 4T1/sh HMGB1 mice. There was no difference in serum levels of HMGB1 among 4T1, 4T1/sh control and 4T1/sh HMGB1 mice. Data are shown as mean ± SD (n = 8). ns: not significantly different from sham. (D) Knockdown of HMGB1 by shRNA in 4T1 BC cells. HMGB1 expression was evaluated by Western analysis in parental 4T1 BC, 4T1/sh control and 4T1/sh HMGB1 cells. HMGB1 expression is successfully reduced in 4T1/sh HMGB1 cells. (E) Expression of molecular pain markers pERK1/2 and pCREB in DRGs of sham, 4T1, 4T1/sh control and 4T1/sh HMGB1 mice. DRGs were harvested after sacrifice at day 15, immediately lysed and subjected to Western analysis. Expression of pERK1/2 and pCREB was increased in DRGs of 4T1 and 4T1/sh control mice compared to sham mice and decreased in DRGs of 4T1/sh HMGB1 mice compared to 4T1 and 4T1/sh control mice. Expression of ERK1/2 and CREB was not changed. (F) Progression of hind-paw mechanical hypersensitivity in tibiae of intact, sham, 4T1, 4T1/sh control and 4T1/sh HMGB1 mice. Sham mice showed hind-paw mechanical hypersensitivity until surgical trauma healed. 4T1 mice withdrew their hind-leg by the force of 3.84 ± 0.84 g (Mean ± SD), while 4T1/sh HMGB1 mice by 7.26 ± 0.43 g (Mean ± SD) at day 10. Data are shown as mean ± SD (n = 8). * Significantly different from intact and sham mice (p < 0.05). # Significantly different from 4T1 and 4T1/sh control mice (p < 0.05). (G) Axogenesis of peripherin+ SNs in the bone marrow of tibiae by immunofluorescent staining. Tibiae were harvested from sham, 4T1, 4T1/sh control and 4T1/sh HMGB1 mice after sacrifice at day 15, fixed, decalcified and processed for subsequent histological analyses. Bone sections were incubated with rabbit anti-peripherin (1:100) antibody overnight at 4 C as primary antibodies, and then with donkey anti-rabbit IgG (1:100) as secondary antibody, followed by Alexa Fluor 488 anti-rabbit IgG (1:1000). Peripherin is a marker for SN. White asterisks in the figures indicate cortical bone. Peripherin+ SNs were increased in the bone marrow of tibiae of 4T1 and 4T1/sh control mice compared to sham mice and decreased in the bone marrow of tibiae of 4T1/sh HMGB1 mice compared to 4T1 and 4T1/sh control mice. Scale bar 250 µm. (H) Quantitative evaluation of peripherin+ SNs in the bone marrow of tibiae seen in Fig. 2G. Peripherin+ fluorescent area and whole bone marrow area in histological sections were quantified under a fluorescent microscope using Neuron J software. Peripherin+ SNs (%) on Y-axis in the figure was calculated as peripherin+ fluorescent area/total bone marrow area ×100. Peripherin+ SNs were increased in 4T1 mice, which was decreased by the treatment with HMGB1 antibody. Data are shown as mean ± SD (n = 8). * Significantly different from sham mice (p < 0.05). # Significantly different from 4T1 and 4T1/sh control mice (p < 0.05).