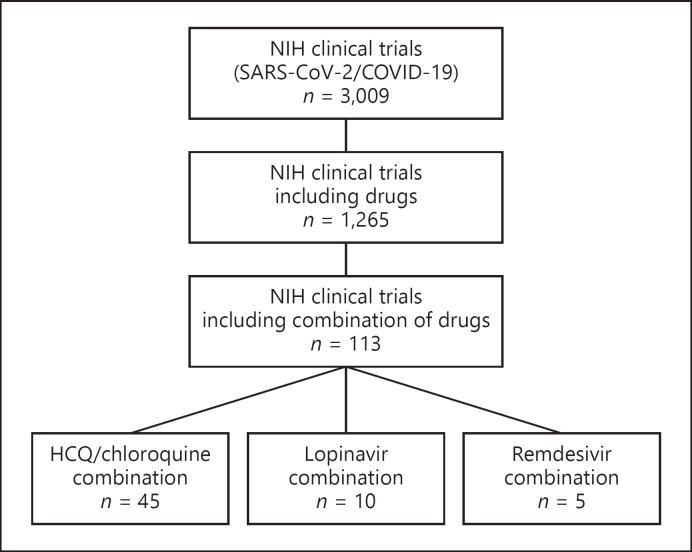
Fig. 2.
Clinical trials using a combination of drugs targeting early replication steps or viral enzymes. An exhaustive revision of the ongoing clinical trials related to SARS-CoV-2 was performed using the database of the National Institutes of Health (NIH) of the United States. The data were accessed from the NIH webserver ClinicalTrials.gov. The search terms were “COVID” or “SARS-CoV-2,” resulting in 3,009 trials. Then, the search parameter drug was applied as a further term, retrieving 1,616 clinical trials. In each search, the trials included those not yet recruiting, recruiting, active, or completed. A visual inspection of each of the 1,616 trials was conducted and only 1,265 included drugs as the main intervention. Moreover, the drug combination trials were also reviewed, and only 113 included a combination of pharmacological therapy for the treatment of COVID-19 patients. The database search was conducted on August 14, 2020. COVID-19, coronavirus disease 2019; HCQ, hydroxychloroquine; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.