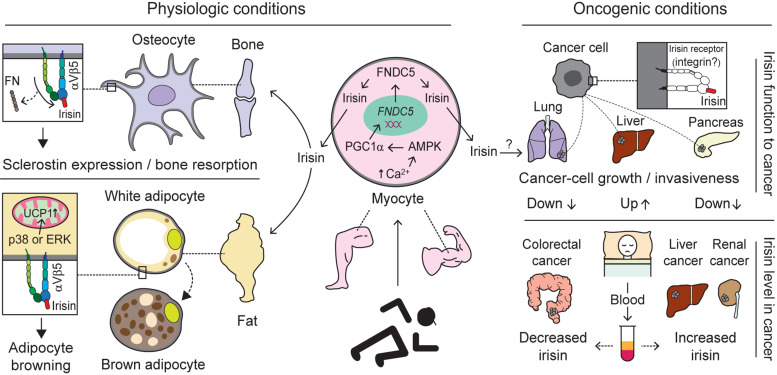
FIGURE 4.
Biological interactions mediated by integrins with irisin. Exercise-induced production and release of irisin from the myocytes of skeletal muscle through activation of the AMPK-PGC1α-FNDC5 axis alters biological pathways via interaction with integrin (Bostrom et al., 2012; Shan et al., 2013; Gamas et al., 2015). Under physiologic conditions, irisin migrated to the bone via blood circulation preferentially, compared to FN, interacts with integrin αVβ5 acting on osteocytes to induce sclerostin expression and bone resorption. Moreover, irisin binding to Vβ5 on adipocytes is believed to facilitate adipocyte browning through the activation of p38 or ERK followed by increases in UCP1 (Zhang et al., 2014; Kim et al., 2018). Under oncogenic conditions, irisin binding to cancer cells is thought to have different impacts, although irisin receptors specific to cancer cells exist remains an open question. Specifically, irisin plays a role in inhibiting the growth or invasiveness of lung- and pancreatic-cancer cells (Shao et al., 2017; Liu et al., 2018), whereas it stimulates liver-cancer cells to promote tumor progression (Shi et al., 2017). In addition, the levels of irisin in blood (serum or plasma) of cancer patients appear to vary depending on which organ is primarily cancerous. For example, increased levels of irisin were found in liver- and renal-cancer patients (Gaggini et al., 2017; Altay et al., 2018), while decreased levels were detected in colorectal-cancer patients (Zhu et al., 2018). This suggests irisin levels may serve as a diagnostic marker for different cancers. AMPK, adenosine monophosphate (AMP)-activated protein kinase; PGC1α, peroxisome proliferator-activated receptor coactivator 1α; FNDC5, fibronectin type III domain-containing protein 5; FN, fibronectin; ERK, extracellular signal-regulated kinase; and UCP1, uncoupling protein 1.