Abstract
Objectives
Human papillomavirus (HPV) vaccination rates among adolescents are lower in rural areas than in urban areas of the United States. The objective of this study was to identify barriers to and facilitators of adolescent HPV vaccination in Montana, a large, primarily rural state.
Methods
Using a mixed-methods design, we integrated quantitative analyses of Montana’s National Immunization Survey–Teen (NIS-Teen) data from 2013-2017 with qualitative data collected at a statewide meeting in October 2018 and from stakeholder interviews conducted from October 2018 through June 2019. Using NIS-Teen data, we identified trends and estimated adjusted prevalence ratios (aPRs) to identify factors associated with vaccine uptake. Using directed content analysis of qualitative data, we identified themes related to vaccine uptake.
Results
In Montana, initiation of the HPV vaccine series among adolescents aged 13-17 increased from 34.4% in 2013 to 65.5% in 2017. We identified 6 themes related to HPV vaccination from qualitative analyses, including medical providers’ recommendation style as a facilitator of vaccination and parental vaccine hesitancy as a barrier to vaccination. In NIS-Teen 2017 data (n = 326 adolescents), receiving a medical provider recommendation was significantly associated with series initiation (aPR = 2.3; 95% CI, 1.5-3.6). Among parents who did not intend to initiate the vaccine series for their adolescent within 12 months (n = 71), vaccine safety was the top concern (aPR = 24.5%; 95% CI, 12.1%-36.9%).
Conclusions
HPV vaccination rates have increased in Montana but remain lower than rates for other adolescent vaccines. Future work should focus on reducing missed opportunities, increasing parents’ knowledge of and confidence in vaccination, and training medical providers on addressing common vaccine concerns.
Keywords: vaccines, adolescent health, rural health, public health, infectious disease, NIS-Teen
Human papillomavirus (HPV) infections are the most common sexually transmitted infection in the United States.1,2 HPV infection can cause several types of cancer, including cervical, penile, oropharyngeal, and anal cancers.3 In 2006, a quadrivalent HPV vaccine was licensed in the United States as a 3-dose series. After licensure, the Advisory Committee on Immunization Practices (ACIP) recommended HPV vaccination for adolescent females; in 2011, the recommendation was expanded to include adolescent males.4 In 2014, a 9-valent HPV vaccine was introduced, and in 2016, the ACIP recommendation on the number of doses in the series changed. Currently, adolescents are recommended to start the 2-dose vaccine series at age 11 or 12, but 3 doses are recommended if the series is initiated at or after age 15.5,6
Nationally, HPV vaccine coverage has lagged behind uptake of other adolescent vaccines. In 2017, the percentage of US adolescents aged 13-17 who had received the tetanus-diphtheria-acellular pertussis (Tdap) vaccine was 88.7%. In contrast, initiation of the HPV vaccine series (ie, receipt of ≥1 HPV vaccine dose) was 65.5%, and completion of the HPV vaccine series was 48.6%, below the Healthy People 2020 goal of 80%.7 Studies have identified wide-ranging barriers to adolescent HPV vaccination, including financial barriers, parental hesitancy about the necessity and safety of the vaccine, and lack of strong recommendations from medical providers.8 Interventions to increase HPV vaccine uptake have focused on addressing these barriers, including providing increased access to immunization services and educating medical providers about the vaccine, including effective communication strategies.9,10
Although HPV vaccination rates among adolescents have been increasing nationally,7 a persistent disparity in vaccination rates exists between rural and urban areas. In 2017, HPV vaccination initiation rates were 10.8 percentage points lower among adolescents residing in rural areas than in urban areas of the United States.7 A recent study reported that among rural US adolescents unvaccinated against HPV, 78.9% had a missed opportunity for vaccination, defined as receipt of another vaccine at age 11 or 12 but not the HPV vaccine during the same health care encounter.11 The National Vaccine Advisory Committee has recommended additional research on barriers to HPV vaccination in rural areas,12 and the National HPV Vaccination Roundtable has prioritized the identification of strategies to increase immunization rates in rural areas as a top research priority.13
Montana is the fourth-largest state in geographic size but 44th in population size, with approximately 1.1 million residents.14 Montana’s current State Health Improvement Plan includes goals to increase rates of early childhood and adolescent vaccination, including HPV, by 2023.15 Similar to US trends, rates of adolescent HPV vaccination in Montana have been lower than rates of adolescent Tdap vaccination, which is required for school attendance in Montana. To inform how Montana may increase HPV vaccination rates, the objectives of our study were to identify current barriers to and facilitators of adolescent HPV vaccine uptake in Montana. We used a mixed-methods approach that integrated analysis of National Immunization Survey–Teen (NIS-Teen) data with qualitative data collected from medical and public health stakeholders.
Methods
Study Overview
We used NIS-Teen public-use data to identify factors associated with adolescent HPV vaccine series initiation in Montana.16 We collected qualitative data at a statewide meeting of organizations involved in HPV prevention and through interviews with medical providers and other public health stakeholders. Our preliminary analyses of NIS-Teen data informed development of semi-structured tools to guide these interviews. We then conducted additional NIS-Teen analyses on the basis of interview findings, thus integrating our quantitative and qualitative methodology.
We used the Vaccine Perceptions, Accountability and Adherence Model by Katz et al17 as a conceptual framework to inform our approach. The model was developed to guide community- and population-level efforts in increasing HPV vaccine series initiation and completion. The model addresses how environmental, structural and sociocultural, and individual adolescent and caregiver factors influence HPV vaccine acceptance.17 We also considered how medical provider factors, including medical providers’ support of and recommendation for the vaccine, influence vaccine uptake in Montana (Table 1).
Table 1.
Factors influencing human papillomavirus vaccine uptake and adherence, modified from the Vaccine Perceptions, Accountability and Adherence Modela
| Factors | Examples |
|---|---|
| Environmental |
|
| Structural and sociocultural |
|
| Adolescent and caregiver |
|
| Medical provider |
|
aModel developed by Katz et al.17
The University of Montana’s Institutional Review Board classified this study as exempt.
Quantitative Data and Analyses
NIS-Teen is a nationally representative survey conducted annually by the Centers for Disease Control and Prevention (CDC) to monitor adolescent vaccination coverage levels at national, state, and some local levels. NIS-Teen methodology is described in detail elsewhere,18 and survey interview tools are publicly available.19 Briefly, NIS-Teen uses random-digit dialing of cell phones and landline telephones, and interviews are conducted with parents or guardians of adolescents aged 13-17. Parents are asked to consent to allowing survey administrators to contact the adolescent’s immunization provider to obtain immunization records. We limited our analyses to adolescents in Montana with medical provider–confirmed immunization data (our only inclusion criterion). Analyses accounted for the complex survey design and incorporated NIS-provided sampling weights.16 NIS-Teen designates whether the adolescent lives in 1 of 3 areas: a metropolitan statistical area (MSA) principal city, an MSA nonprincipal city (ie, suburban), or a non-MSA. Similar to other studies using NIS-Teen data,11 our study refers to non-MSAs as rural areas and MSA principal cities as urban areas. Montana does not have any MSA nonprincipal cities.
Using NIS-Teen data available in CDC’s TeenVaxView online tool,20 we examined trends in the initiation of the adolescent HPV vaccine series during 2013-2017 by sex and by whether the adolescent lived in a rural or urban area. We also examined rates of Tdap and meningococcal vaccination, which are also recommended by the ACIP for the same age group. Using NIS-Teen public-use files,21 we examined overall rates of HPV vaccine series initiation in 2013-2017 and conducted bivariate and multivariable analyses of factors associated with series initiation in 2017. For these analyses, we combined or masked some categories to report results only from subgroups with at least 30 people.16 We considered the following factors: sex (male or female), maternal education (≥some post–high school or ≤high school graduate), type of health insurance (Medicaid, private health insurance only, other health insurance, uninsured), and annual family household income (≤$75 000 or >$75 000). We also examined data on the adolescent’s race/ethnicity and whether the adolescent had received a checkup in the previous 2 years; however, we did not report these data because of small cell sizes. We reported characteristics of Montana adolescents who did and did not initiate the HPV vaccine series, and we compared these characteristics using linear regression and Rao–Scott χ2 tests. We estimated prevalence ratios for series initiation based on demographic, socioeconomic, and health access factors in unadjusted and multivariable models. Statistical tests were 2-sided with an α level of .05.
On the basis of results from stakeholder interviews, we also examined data from 2 additional survey questions available in NIS-Teen public-use files: parents’ report that a medical provider had recommended the HPV vaccine for their adolescent child (NIS-Teen survey question: “Has a doctor or other health care professional ever recommended that [TEEN] receive HPV shots?”) in survey years 2013-2017, and parent-reported reasons for not initiating the HPV vaccine series in 2017 (NIS-Teen survey question: “What is the main reason [TEEN] will not receive HPV shots in the next 12 months?”; parent response coded as 1 of 16 potential reasons).19
We conducted quantitative analyses from October 2018 through June 2019. We analyzed NIS-Teen public-use files with SAS version 9.4 (SAS Institute Inc).
Qualitative Data and Analyses
We collected qualitative data on barriers to and facilitators of adolescent HPV vaccination in Montana in 2 phases. The first phase consisted of collecting qualitative data consisting of a transcript from a recording of a single-session statewide HPV vaccination stakeholder meeting hosted by the American Cancer Society in Helena, Montana, in October 2018. The purpose of the half-day meeting was to convene stakeholders in adolescent HPV vaccine delivery in Montana to identify opportunities for enhanced and ongoing partnership and coordination. Two study team members (S.R.N. and E.C.) were both participants in and observers of this meeting. Ten additional meeting participants were representatives from the state health department, health insurance plans, medical provider groups, and nonprofit organizations working in HPV prevention. The meeting agenda included reviewing current data on HPV vaccination, ongoing challenges that had already been identified in the state, current activities supporting vaccine promotion, and opportunities to increase HPV vaccine coverage.
The second phase of qualitative data collection involved conducting one-on-one or small group interviews with key stakeholders, including medical providers. We interviewed a range of medical providers whose scope of practice included independent care of adolescent patients, including family medicine physicians, pediatricians, advanced practice nurses, and physician assistants, and public health nurses who provide immunizations at local health departments. We sought input from medical providers from eastern and western Montana, as well as from both rural and more populated areas. Most medical providers in larger Montana cities care for rural patients who travel from surrounding counties. We identified medical providers from suggestions of participants in the October 2018 stakeholder meeting, from a statewide adolescent health meeting hosted by the state health department later that month, and from suggestions from interviewees.
We also interviewed other key stakeholders who were not direct medical providers, including members of the state public health department, rural health–focused nonprofit organizations, and health insurance plans. Some of these stakeholders also participated in the October 2018 meeting, and we conducted one-on-one interviews to solicit additional information. We contacted potential stakeholder interviewees by telephone or email, and we conducted interviews by telephone or in person. For the interviews, we developed a semi-structured tool with open-ended questions. Interviews of medical providers started with general questions about clinical processes and workflows for adolescent immunizations. Interviews of other stakeholders started by inquiring about organizational activities related to adolescent HPV vaccination. Then, both types of interviews included questions about stakeholders’ knowledge of and experiences with barriers to HPV vaccination in Montana, ideas for opportunities to increase vaccine uptake, and, if applicable, whether and how other stakeholders had been included in their vaccination efforts. We approached 16 people for interviews and 13 agreed to participate, including 2 people who had also participated in the HPV vaccination stakeholder meeting. Interviews lasted 20 to 50 minutes and were conducted from October 2018 through June 2019. All interviews were one-on-one except for one with 4 public health nurses conducted as a small group session.
We audio-recorded and transcribed the HPV vaccination stakeholder meeting and stakeholder interviews. We used a directed content analysis approach, whereby we documented anticipated themes before analysis.22 These anticipated themes were based on previous research and knowledge of barriers to and facilitators of HPV vaccination that were identified in other settings.8,9 Two study team members (E.C. and T.S.) independently conducted line-by-line open coding of the transcribed meeting and interviews, assigning data to 19 codes (subthemes) within 7 broader coding categories (themes). The study team held regular meetings to review coding progress, identify emerging themes and subthemes, compare notes, and recode and organize data into more selective and focused categories.23 This recoding process resulted in 6 themes and 13 subthemes. We tabulated these results with representative quotes from stakeholders.
We conducted qualitative analyses from January through August 2019 with NVivo version 12 software (QSR International).
Results
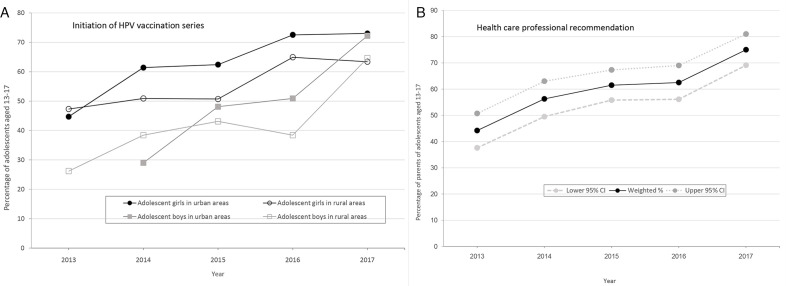
From 2013 to 2017, HPV vaccine series initiation rates among female and male Montana adolescents aged 13-17 increased in both rural and urban areas of Montana (Figure A). From 2016 to 2017, the rate increased from 55.3.% to 65.5%; the large increase was partially due to increasing vaccination rates among adolescent boys in both urban areas (from 50.9% to 72.2%) and rural areas (from 38.4% to 64.6%). According to 2017 NIS-Teen data, 72.6% of adolescents in urban areas of Montana had initiated the series, whereas only 64.0% of adolescents in rural areas of Montana had initiated the series. Although HPV vaccination rates have been steadily increasing in Montana, missed opportunities occurred: by 2017, 90.4% of Montana adolescents had received a Tdap vaccine and 71.2% had received a meningococcal vaccine, whereas 65.5% had received at least 1 HPV vaccine dose.
Figure.
Trends in initiation of human papillomavirus (HPV) vaccine series and medical provider recommendations among adolescents aged 13-17, National Immunization Survey–Teen (NIS-Teen), Montana, 2013-2017. (A) Percentage of adolescents who initiated HPV vaccination series, by sex and rural or urban residence. NIS-Teen designates whether adolescents live in a metropolitan statistical area (MSA) principal city, an MSA nonprincipal city (ie, suburban), or a non-MSA. In this study, MSA principal city areas are considered urban and non-MSAs are considered rural. Montana does not have any MSA nonprincipal city areas. Data obtained from the Centers for Disease Control and Prevention’s TeenVaxView online tool.20 (B) Percentage of parents or guardians who responded yes to the question, “Had or has doctor or other health care professional ever recommended that teen receive HPV shots?” Data obtained from NIS-Teen’s 2013-2017 public-use data files.19 Because of a change in how adequate provider data for immunization records were assessed starting with the 2014 NIS-Teen, vaccine series initiation rates from 2013 may not be directly comparable with rates from later years.16
In univariate analyses of NIS-Teen 2017 data (n = 326), we found no significant differences between adolescents who did and did not initiate the HPV vaccine series by age, sex, mother’s education, type of health insurance, or annual family household income (Table 2). In multivariable modeling of NIS-Teen 2017 data, 2 variables were significantly associated with HPV vaccine initiation: adolescent receipt of Tdap vaccination (adjusted prevalence ratio [aPR] = 3.3; 95% CI, 1.4-8.1) and a parent or guardian reporting that a medical provider had recommended the HPV vaccine for the child (aPR = 2.3; 95% CI, 1.5-3.6) (Table 3).
Table 2.
Characteristics of adolescents aged 13-17 who did and did not initiate the human papillomavirus (HPV) vaccine series, Montana, National Immunization Survey–Teen (NIS-Teen), 2017a
| Characteristic | Total (N = 326) | Initiated the HPV vaccine series (n = 209) | Did not initiate the HPV vaccine series (n = 117) | P valueb |
|---|---|---|---|---|
| Weighted % (95% CI) of total | — | 65.5 (59.1-71.8) | 34.5 (28.2-40.9) | — |
| Age of adolescent, weighted mean (95% CI), years | 15.0 (14.8-15.2) | 15.1 (14.8-15.3) | 14.8 (14.5-15.1) | .18 |
| Sex | ||||
| Female | 150 (48.8 [42.2-55.5]) | 100 (48.2 [39.9-56.4]) | 50 (50.2 [38.9-61.4]) | .78 |
| Male | 176 (51.2 [44.5-57.8]) | 109 (51.8 [43.6-60.1]) | 67 (49.8 [38.6-61.1]) | |
| Maternal educationc | ||||
| ≥Some post–high school | 163 (55.9 [49.4-62.4]) | 105 (58.3 [50.4-66.3]) | 58 (51.3 [40.1-62.5]) | .31 |
| ≤High school | 163 (44.1 [37.6-50.6]) | 104 (41.7 [33.7-49.6]) | 59 (48.7 [37.5-59.9]) | |
| Type of health insuranced | ||||
| Medicaid | 117 (40.8 [34.1-47.5]) | 74 (42.7 [34.4-51.1]) | 43 (37.1 [26.0-48.2]) | .33 |
| Private health insurance only | 168 (46.6 [40.0-53.2]) | 108 (44.6 [36.4-52.7]) | 60 (50.4 [39.1-61.7]) | |
| Annual family household income, $e | ||||
| ≤75 000 | 184 (64.4 [58.2-70.5]) | 112 (64.0 [56.4-71.6]) | 72 (65.1 [54.6-75.6]) | .87 |
| >75 000 | 134 (35.6 [29.5-41.8]) | 92 (36.0 [28.4-43.6]) | 42 (34.9 [24.4-45.4]) | |
aData source: Centers for Disease Control and Prevention.19 Analyses were limited to adolescents for whom adequate health care provider data on immunization history were available. Analyses accounted for the complex survey design and incorporated NIS-Teen–provided sampling weights. Data for race/ethnicity and whether the child had a checkup in previous 2 years are not shown because of small cell sizes. All values are no. (weighted % [95% CI]) unless otherwise indicated.
bFor the continuous variable of age, P values were determined by linear regression. For categorical variables, the Rao–Scott χ2 test was used to determine P values. P < .05 was considered significant.
c“≥Some post–high school” includes mothers with some post–high school education and college graduates. “≤High school” includes high school graduates and mothers with <12 years of education.
dData for uninsured and other health insurance categories are not shown because of small cell sizes.
eData on annual family household income were missing for 8 people.
Table 3.
Factors associated with human papillomavirus (HPV) vaccine series initiation among adolescents aged 13-17, Montana, National Immunization Survey–Teen, 2017a
| Factor | Sample no. | Weighted % (95% CI) | PR (95% CI) for HPV vaccine series initiation | |
|---|---|---|---|---|
| Unadjusted | Adjustedb | |||
| Adolescent received a Tdap vaccine after 11th birthday | ||||
| Yes | 292 | 90.4 (86.5-94.3) | 3.8 (1.8-8.0) | 3.3 (1.4-8.1) |
| No | 34 | 9.6 (5.7-13.5) | Reference | Reference |
| Parent/guardian received a medical provider’s recommendation to vaccinate adolescent against HPVc | ||||
| Yes | 245 | 78.9 (73.3-84.6) | 2.4 (1.6-3.8) | 2.3 (1.5-3.6) |
| No | 63 | 21.1 (15.4-26.7) | Reference | Reference |
Abbreviations: PR, prevalence ratio; Tdap, tetanus-diphtheria-acellular pertussis.
aData source: Centers for Disease Control and Prevention.19
bAdjusted for adolescent’s age, sex, maternal education level, health insurance type, annual family household income, race/ethnicity, whether the child had a checkup in the previous 2 years, and the other factor listed in the table.
cThirteen people answered that they did not know whether they had received a medical provider recommendation to vaccinate, and data were missing for 5 people; these 18 people were excluded from analysis.
Eleven medical providers and 10 public health stakeholders participated in the statewide HPV meeting or interviews. We found 6 main themes on barriers to and facilitators of adolescent HPV vaccination in Montana (Table 4). Two themes—medical providers’ recommendation style and parental vaccine hesitancy—were the most commonly referenced. Medical providers’ recommendation style emerged as a facilitator of HPV vaccination. Medical providers reported support of and confidence in the vaccine, and many discussed using a presumptive style of recommending the vaccine (ie, assuming the parent intends to vaccinate) and offering the HPV, meningococcal, and Tdap vaccines together. Several participants perceived that parents were more receptive to the HPV vaccine when it was discussed as a tool for cancer prevention rather than a tool for sexually transmitted infection prevention. Public health stakeholders shared information on previous and ongoing efforts to educate health professionals in Montana about the HPV vaccine through in-person and online training. Analysis of NIS-Teen data supported the qualitative finding of provider support for and recommendation of the HPV vaccine: the percentage of Montana parents who reported receiving a recommendation from a medical provider to vaccinate their adolescent against HPV increased from 44.2% (95% CI, 37.6%-50.7%) in 2013 to 75.0% (95% CI, 69.1%-81.0%) in 2017 (Figure B).
Table 4.
Themes, subthemes, and representative quotes on barriers to and facilitators of adolescent human papillomavirus (HPV) vaccination, identified from qualitative data collected from medical providers (n = 11) and public health stakeholders (n = 10), Montana, October 2018–June 2019
| Theme | Subthemes | Barrier or facilitator | Representative quote(s) |
|---|---|---|---|
| Medical providers’ recommendation style |
|
Facilitator |
|
| Parental vaccine hesitancy |
|
Barrier |
|
| Adolescent engagement in vaccine discussions |
|
Both | People bargain with their kids . . . to get them in here, [and] say, “Well, you’re only going to get one shot today.” Somehow, educating them that there are 3 recommended vaccines . . . would be helpful. [Public health nurse B] |
| Policies and laws |
|
Both | Teens can get birth control on their own, but they cannot get an HPV vaccine. That’s a frustration . . . because they ask for it and we can’t give it to them. [Public health nurse A] |
| Social determinants of health |
|
Barrier | We have providers all over the state that are VFC [Vaccines for Children] providers. However, . . . in some rural areas . . . they only have the public health department as their VFC provider. [Public health stakeholder A] |
| Irregular adolescent well care | Not applicable | Barrier | Adolescents do not seek care for well visits. . . [so] we have to do our vaccines on acute visits. That’s when it has to be done because that’s when they are there in your office. [Physician B] |
Medical providers identified parental vaccine hesitancy as a barrier to vaccination (Table 4). Medical providers indicated that parents more often had questions about the HPV vaccine than about other vaccines recommended for adolescents. Medical providers reported that parents’ concerns ranged from questions about vaccine safety or the recommended age to initiate vaccination to concerns about vaccine misinformation (such as the vaccine causing infertility or sudden death) in social media. In 2017 NIS-Teen data, among parents of unvaccinated adolescents who did not intend to vaccinate within the next 12 months (n = 71), the top reason cited for nonintent to vaccinate was safety/side effect concerns (24.5%; 95% CI, 12.1%-36.9%).
Four additional themes were adolescent engagement in vaccine discussions, policies and laws, social determinants of health, and irregular adolescent well care (Table 4). Some medical providers talked about engaging adolescents in vaccine discussions, while others focused their conversation on parents or guardians. Interview participants also discussed how adolescents are engaged by parents, in particular how parent–adolescent dynamics affect vaccine decisions. Policies and laws were recognized as a facilitator of and barrier to vaccination. Requiring adolescents to have a Tdap vaccine for school attendance was seen as an opportunity for providing other vaccines. However, some stakeholders reported that the mandatory requirement sometimes led to perceptions among parents that the Tdap vaccine was more important than other adolescent vaccines, including HPV. In addition, laws limiting adolescent consent for vaccination were cited as a barrier. Social determinants of health, including barriers to access in extremely rural counties, were discussed, as were barriers among unique populations, such as adolescents in foster care. Finally, irregular adolescent well-care emerged as a barrier, with some stakeholders perceiving that adolescents do not come in as regularly as younger children for well-child visits.
Discussion
Adolescent HPV vaccination rates are rising in Montana, with 2017 HPV vaccine series initiation mirroring the national average of 65.5%.7 However, HPV vaccine uptake was higher in urban areas than in rural areas of Montana, and rates of HPV vaccination lagged behind rates of other adolescent vaccinations. We identified several opportunities to continue to increase HPV vaccine uptake in this large and primarily rural state.
Increasing medical provider recommendation for the HPV vaccine presumably contributed to Montana’s increasing vaccination rates. In quantitative analyses, we observed an increase over time in the percentage of Montana parents of adolescents who reported receiving a medical provider recommendation to vaccinate against HPV. Consistent with other studies,9,24 our study found that receiving a medical provider recommendation was associated with adolescents initiating the HPV vaccine series. In qualitative analyses, public health and medical stakeholders communicated support of and confidence in the vaccine as a tool for cancer prevention. These findings align with evidence supporting the importance of strong and consistent vaccine recommendations from medical providers,10,25 as well as the effectiveness of messaging that frames the HPV vaccine as a means of cancer prevention rather than prevention of sexually transmitted infection.26
Many barriers to vaccination identified in this study are not unique to Montana. Parental vaccine hesitancy, including concerns about the vaccine’s safety and necessity, was identified as a barrier in other national27 and state investigations, including states with large rural populations, such as Alabama and South Carolina.28,29 However, some barriers identified by stakeholders in our study are unique to a large, rural state such as Montana, including access to health care in remote areas. Strategies to increase HPV vaccination rates may work differently in states with largely rural populations than in states with populations focused in urban centers. For example, public awareness campaigns may have less reach in rural areas than in urban areas. Determining which interventions work to increase HPV vaccine uptake in rural areas in the United States is a research priority of the American Cancer Society’s National HPV Vaccination Roundtable.13 We encourage the development and testing of such interventions in states such as Montana.
From this study, we identified strategies to continue to support increasing HPV vaccination rates among adolescents in Montana. One strategy is to focus on reducing the number of missed vaccination opportunities. Montana state law requires Tdap vaccination before seventh grade, and efforts should continue to support offering all 3 recommended vaccines (Tdap, HPV, and meningococcal) when an adolescent is offered the Tdap vaccine. However, some stakeholders indicated that parents are not always aware that these 3 vaccines, along with annual influenza vaccination, are recommended for adolescents. Similar to ongoing work to educate parents about newborn immunizations before the birth of their child,30 efforts to educate parents about adolescent vaccines before ages 11 or 12 should be explored. Moreover, our results point to the need for additional work in increasing public confidence in vaccines. For this, we encourage parental and patient input on when, where, and how they would like to receive information about HPV vaccination. Finally, continued education about HPV vaccination among health care and public health professionals in Montana should be sustained, including training on anticipating and responding to common parental concerns.
Limitations
Our study had several limitations. First, we did not obtain qualitative input from parents, guardians, or adolescents. These groups could provide additional insight into the barriers to and facilitators of HPV vaccination, and we have initiated work in western Montana to engage these stakeholder groups. Second, all medical providers who participated in the interviews supported use of the HPV vaccine; medical providers less supportive of the vaccine may not have been interested in participating or were not referred to our study. Had we received input from medical providers who did not support HPV vaccination, our qualitative findings may have differed from those described here. Third, we focused on HPV vaccine series initiation; however, series completion is crucial for disease prevention. Reminder/recall programs can improve completion rates for adolescent immunizations,31 and research is needed to understand whether rural clinics are using these or other strategies for HPV vaccine series completion.
Conclusions
By integrating quantitative and qualitative methods, we identified facilitators of and barriers to HPV vaccination in Montana. Although HPV vaccination rates are on the rise in this large, rural state, sustained work is needed to continue to increase vaccine uptake. Future strategies should focus on reducing the number of missed opportunities to provide HPV vaccine, increasing public confidence in the vaccine, and training health care professionals to respond to common vaccine concerns.
All analyses, interpretations, and conclusions using the NIS-Teen public-use data source are those of the authors (recipient of the data source) and not of the Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases, which is responsible only for the initial data.
Footnotes
Authors’ Note: Preliminary findings from this work were presented at the 2019 American Public Health Association Annual Meeting, Philadelphia, Pennsylvania.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Mountain West Clinical Translational Research–Infrastructure Network under a grant from National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health under award number 2U54GM104944. Drs Newcomer and Caringi were also supported by an NIGMS Center for Biomedical Research Excellence award (1P20GM130418).
ORCID iD
Sophia R. Newcomer, PhD, MPH https://orcid.org/0000-0002-6664-3266
References
- 1. Satterwhite CL., Torrone E., Meites E. et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187-193. 10.1097/OLQ.0b013e318286bb53 [DOI] [PubMed] [Google Scholar]
- 2. McQuillan GM., Kruszon-Moran D., Markowitz LE., Unger ER., Paulose-Ram R. Prevalence of HPV in adults aged 18-69: United States, 2011-2014. NCHS Data Brief. 2017;280:1-8. [PubMed] [Google Scholar]
- 3. Tota JE., Chevarie-Davis M., Richardson LA., Devries M., Franco EL. Epidemiology and burden of HPV infection and related diseases: implications for prevention strategies. Prev Med. 2011;53(suppl 1):S12-S21. 10.1016/j.ypmed.2011.08.017 [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705-1708. [PubMed] [Google Scholar]
- 5. Robinson CL., Bernstein H., Romero JR., Szilagyi P. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2019. MMWR Morb Mortal Wkly Rep. 2019;68(5):112-114. 10.15585/mmwr.mm6805a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meites E., Kempe A., Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405-1408. 10.15585/mmwr.mm6549a5 [DOI] [PubMed] [Google Scholar]
- 7. Walker TY., Elam-Evans LD., Yankey D. et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2017 [published correction appears in MMWR Morb Mortal Wkly Rep. 2018;67(41):1164]. MMWR Morb Mortal Wkly Rep. 2018;67(33):909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holman DM., Benard V., Roland KB., Watson M., Liddon N., Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76-82. 10.1001/jamapediatrics.2013.2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smulian EA., Mitchell KR., Stokley S. Interventions to increase HPV vaccination coverage: a systematic review. Hum Vaccin Immunother. 2016;12(6):1566-1588. 10.1080/21645515.2015.1125055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brewer NT., Hall ME., Malo TL., Gilkey MB., Quinn B., Lathren C. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics. 2017;139(1):pii:e20161764. 10.1542/peds.2016-1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams CL., Walker TY., Elam-Evans LD. et al. Factors associated with not receiving HPV vaccine among adolescents by metropolitan statistical area status, United States, National Immunization Survey–Teen, 2016-2017. Hum Vaccin Immunother. 2020;16(3):562-572. 10.1080/21645515.2019.1670036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Vaccine Advisory Committee Strengthening the effectiveness of national, state, and local efforts to improve HPV vaccination coverage in the United States: recommendations from the National Vaccine Advisory Committee. Public Health Rep. 2018;133(5):543-550. 10.1177/0033354918793629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reiter PL., Gerend MA., Gilkey MB. et al. Advancing human papillomavirus vaccine delivery: 12 priority research gaps. Acad Pediatr. 2018;18(2 suppl):S14-S16. 10.1016/j.acap.2017.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Population Review Montana population 2019. Accessed April 26, 2020 http://worldpopulationreview.com/states/montana-population
- 15. Montana Department of Public Health and Human Services Montana State Health Improvement Plan 2019-2023. February 2019. Accessed April 26, 2020 https://dphhs.mt.gov/Portals/85/ahealthiermontana/2019SHIPFinal.pdf
- 16. Centers for Disease Control and Prevention National Immunization Survey–Teen: a user’s guide for the 2017 public-use data file. Accessed April 26, 2020 https://www.cdc.gov/vaccines/imz-managers/nis/downloads/NIS-TEEN-PUF17-DUG.pdf
- 17. Katz IT., Ware NC., Gray G., Haberer JE., Mellins CA., Bangsberg DR. Scaling up human papillomavirus vaccination: a conceptual framework of vaccine adherence. Sex Health. 2010;7(3):279-286. 10.1071/SH09130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jain N., Singleton JA., Montgomery M., Skalland B. Determining accurate vaccination coverage rates for adolescents: the National Immunization Survey–Teen 2006. Public Health Rep. 2009;124(5):642-651. 10.1177/003335490912400506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Centers for Disease Control and Prevention NIS-Teen data and documentation for 2015 to present. Accessed April 26, 2020 https://www.cdc.gov/vaccines/imz-managers/nis/datasets-teen.html
- 20. Centers for Disease Control and Prevention TeenVaxView. Accessed April 26, 2020 https://www.cdc.gov/vaccines/imz-managers/coverage/teenvaxview
- 21. US Department of Health and Human Services, National Center for Immunization and Respiratory Diseases The 2017 National Immunization Survey–Teen. Centers for Disease Control and Prevention; 2018. [Google Scholar]
- 22. Hsieh H-F., Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288. 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- 23. Charmaz K. Constructing Grounded Theory. 2nd ed Sage Publications; 2014. [Google Scholar]
- 24. Gerend MA., Shepherd MA., Lustria MLA., Shepherd JE. Predictors of provider recommendation for HPV vaccine among young adult men and women: findings from a cross-sectional survey. Sex Transm Infect. 2016;92(2):104-107. 10.1136/sextrans-2015-052088 [DOI] [PubMed] [Google Scholar]
- 25. Chung Y., Schamel J., Fisher A., Frew PM. Influences on immunization decision-making among US parents of young children. Matern Child Health J. 2017;21(12):2178-2187. 10.1007/s10995-017-2336-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shah PD., Calo WA., Gilkey MB. et al. Questions and concerns about HPV vaccine: a communication experiment. Pediatrics. 2019;143(2):e20181872. 10.1542/peds.2018-1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beavis A., Krakow M., Levinson K., Rositch AF. Reasons for lack of HPV vaccine initiation in NIS-Teen over time: shifting the focus from gender and sexuality to necessity and safety. J Adolesc Health. 2018;63(5):652-656. 10.1016/j.jadohealth.2018.06.024 [DOI] [PubMed] [Google Scholar]
- 28. Dilley SE., Peral S., Straughn JM., Scarinci IC. The challenge of HPV vaccination uptake and opportunities for solutions: lessons learned from Alabama. Prev Med. 2018;113:124-131. 10.1016/j.ypmed.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cartmell KB., Young-Pierce J., McGue S. et al. Barriers, facilitators, and potential strategies for increasing HPV vaccination: a statewide assessment to inform action. Papillomavirus Res. 2018;5:21-31. 10.1016/j.pvr.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salmon DA., Limaye RJ., Dudley MZ. et al. MomsTalkShots: an individually tailored educational application for maternal and infant vaccines. Vaccine. 2019;37(43):6478-6485. 10.1016/j.vaccine.2019.08.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Szilagyi PG., Albertin C., Humiston SG. et al. A randomized trial of the effect of centralized reminder/recall on immunizations and preventive care visits for adolescents. Acad Pediatr. 2013;13(3):204-213. 10.1016/j.acap.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]