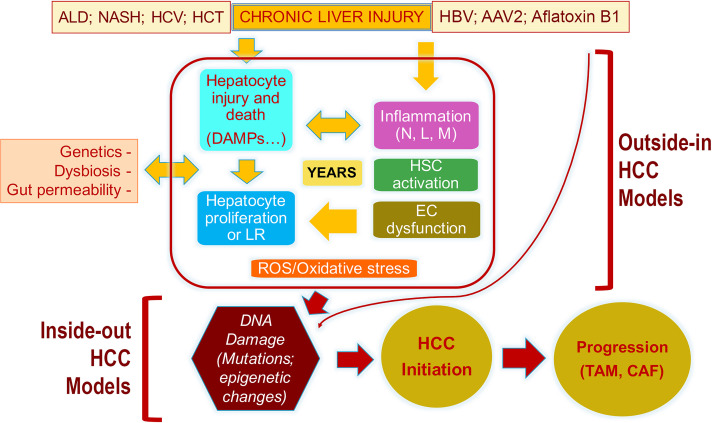
Figure 1.
The pathogenesis of HCC and the descriptions of events captured by the “outside-in” and the “inside-out” models of HCC development. Because of any of the noted chronic injuries to the liver (ALD, alcoholic liver disease; NASH, nonalcoholic steatohepatitis; HCV, hepatitis C virus; HCT, hemochromatosis), there is hepatocyte injury, release of DAMPs, inflammation (N, neutrophils; L, lymphocytes; M, macrophages), hepatic stellate cell (HSC) activation, and endothelial cell (EC) dysfunction, all of which result in the presence of reactive oxygen species (ROS) and oxidative stress. Loss of hepatocyte viability or differentiation also induces hepatocyte replication, which in the presence of ROS/oxidative stress is prone to DNA damage and, eventually, mutations or misexpression of genes. Any such alteration that provides significant growth and survival advantage to a hepatocyte is really the initiation of malignant transformation and HCC development. Once HCC develops, it creates its own microenvironment in the form of cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs), which help in further perpetuation of the tumor. There are models that are more relevant in studying events leading up to the transformation and are referred to as “outside-in” models as these address the microenvironmental changes that are upstream of DNA damage and mutations in the hepatocyte. There are models that utilize expression of sets of genes (normal or mutant) in a subset of normal hepatocytes, which lead to tumor initiation and growth and are referred to as “inside-out” models as the expression of these sets of genes/shRNA is sufficient to induce clinically relevant HCC quite promptly without the need for any previous injury or fibrosis. This suggests that once such DNA aberrations occur in a hepatocyte and the cell is transformed, it does not require any additional cues from the microenvironment to grow and propagate, and the tumor then creates its own microenvironment, which is optimal for its development.