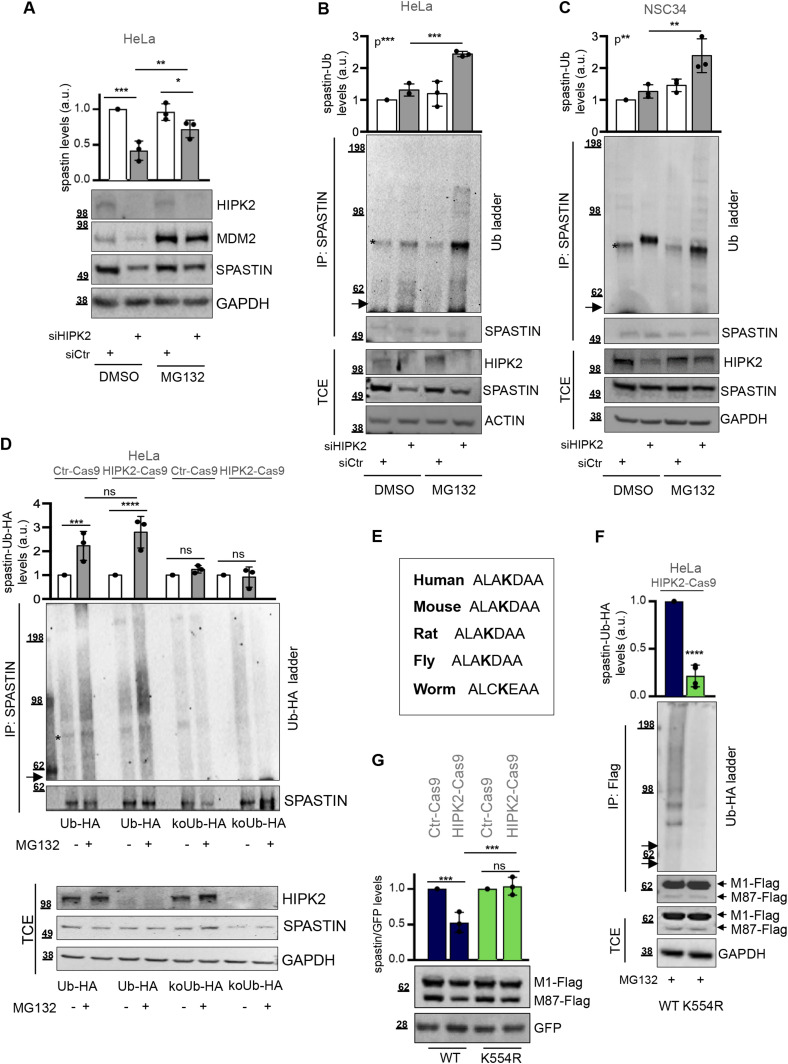
Figure 2. HIPK2 regulates spastin via proteasomal degradation through K554 polyubiquitination.
(A) Representative Western blot (WB) of HeLa cells transfected as in Fig 1A and lysed 96 h posttransfection and 8 h after treatment with 20 μM MG132 or its solvent DMSO. MDM2 stabilization has shown as MG132 positive control. Statistical differences, ANOVA test. (B) HeLa cells were transfected as in Fig 1A and harvested 96 h posttransfection and 8 h after treatment with 20 μM MG132 or DMSO. Total cell extract (TCE) was analysed by WB for the indicated proteins and immunoprecipitated with anti-spastin Ab. IPs were analysed by WB with anti-Ub and anti-spastin Abs. The arrow indicates the position of the unmodified spastin and the asterisk indicates a nonspecific band. The intensity of spastin-Ub ladder was normalized by the intensity of spastin band in IP and reported relative to siCtr DMSO–treated cells as mean ± SD (n = 3). Statistical differences, ANOVA test. (C) NSC34 cells were transfected as in Fig 1B and harvested 5 d posttransfection and 8 h after treatment with 20 μM MG132. TCE was analysed by WB for the indicated proteins and immunoprecipitated with anti-spastin Ab. IPs were analysed by WB with anti-Ub and anti-spastin Abs. The arrow indicates the position of the unmodified spastin and the asterisk indicates a specific band. The intensity of spastin-Ub ladder was calculated and reported as in Fig 2B. Statistical differences, ANOVA test. (D) HeLa Ctr-Cas9 and HIPK2-Cas9 cells were transfected with vectors expressing HA-tagged Ub-WT (Ub-HA) or its derivative KoUb-HA (i.e., Ub with all lysines mutated in arginines) and treated 24 h posttransfection with 20 μM MG132 or DMSO for 8 h. TCEs were analysed as in Fig 2B. IPs were analysed by WB with anti-HA and anti-spastin Abs. The arrow indicates the position of the unmodified spastin and the asterisk indicates a nonspecific band. The intensity of spastin-Ub-HA ladder was normalized by the intensity of spastin band in IP and reported relative to the correspondent DMSO-treated cells as mean ± SD (n = 3). Statistical differences, ANOVA test. (E) Spastin amino acid sequence encompassing the K554 is reported for indicated organisms. Fly = Drosophila melanogaster; worm = Caenorhabditis elegans. (F) HIPK2-Cas9 HeLa cells were transfected with vectors expressing flag-myc–tagged spastin-WT or spastin-K554R in combination with the vector expressing HA-tagged Ub-WT and treated 24 h posttransfection with 20 μM MG132 for 8 h. TCE were analysed by WB and immunoprecipitated with anti-Flag Ab (mouse Ab by Origene Technologies). IPs were analysed by WB with anti-HA and anti-Flag Ab (rabbit Ab by Sigma-Aldrich). The arrows indicate the position of the unmodified spastin isoforms. The intensity of spastin-Ub-HA ladder was normalized by the intensity of spastin bands in IP and reported as mean ± SD (n = 4). Statistical difference, unpaired t test. (G) HIPK2-Cas9 and Ctr-Cas9 HeLa cells were transfected with vectors expressing spastin-WT or spastin-K554R in combination with peGFP vector at 10:1 molar ratio and analysed by WB 24 h posttransfection. GFP expression was used as internal control for transfection efficiency. Representative WB is shown. The intensity of spastin-Flag bands was normalized by the intensity of GFP and reported relative to correspondent Ctr-Cas9 control cells. Statistical differences, ANOVA test.
Source data are available for this figure.