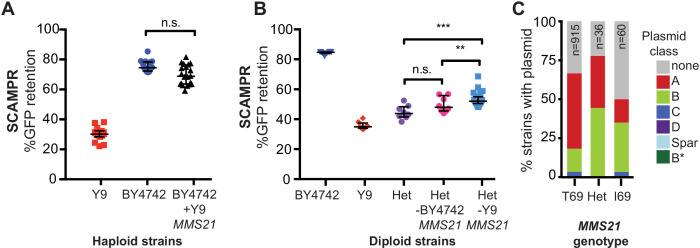
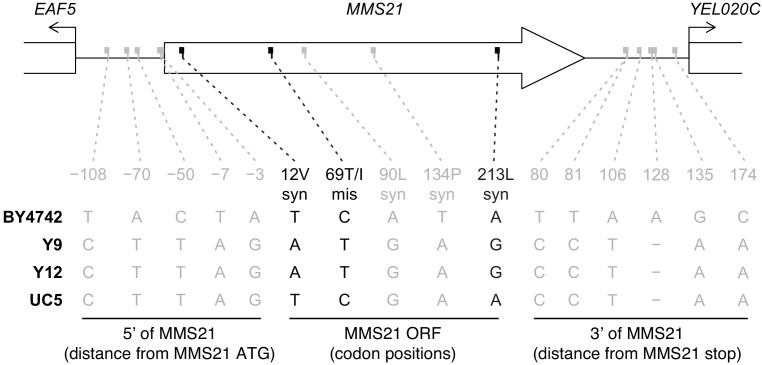
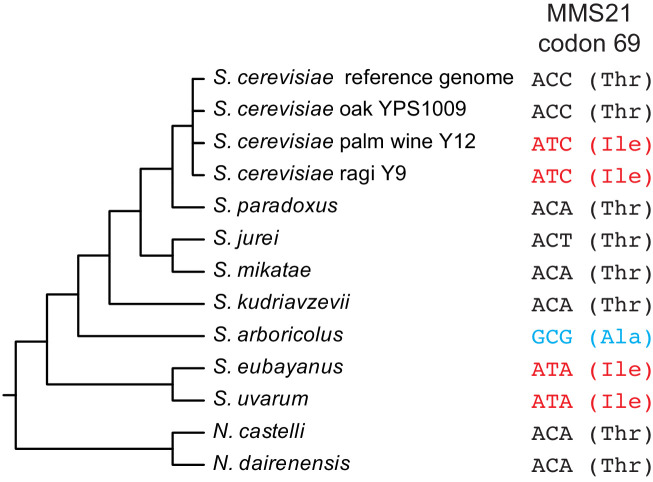
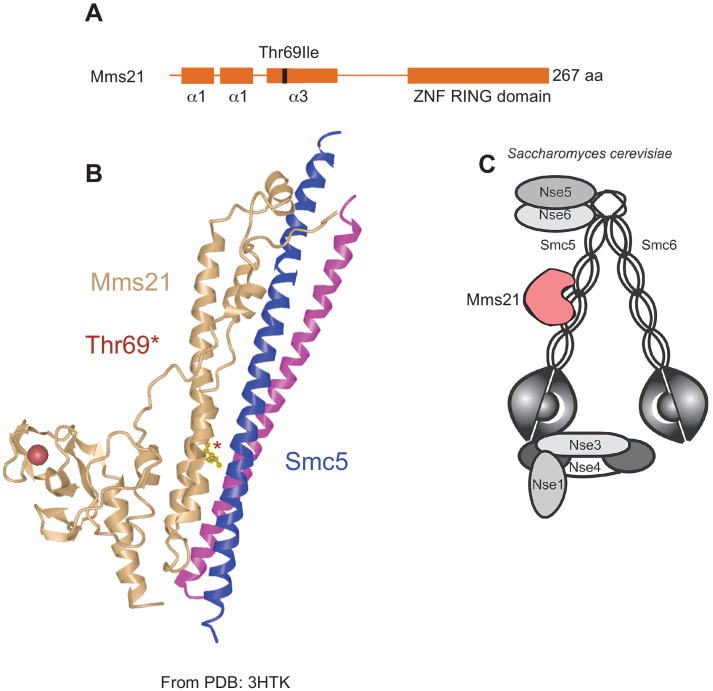
Figure 5. A single SNP in Y9 MMS21 contributes to the plasmid instability phenotype.
(A) Introduction of the Y9 MMS21 allele into BY4742 haploid cells is not sufficient to significantly lower plasmid instability. (B) However, removal of the Y9 MMS21 allele but not the BY4742 MMS21 allele increases plasmid stability in BY4742/Y9 heterozygous diploids, showing that the Y9 allele of MMS21 plays an important role in the Y9 plasmid instability phenotype. **p<0.001, Kruskal-Wallis test, n.s. = not significant. (C) Plasmid prevalence (by plasmid class) for each MMS21 Thr9Ile genotype within 1011 sequenced S. cerevisiae strains. Plasmid data and genotypes from Peter et al., 2018. Strains with the Y9 MMS21 allele (I69) have a lower frequency of harboring 2μ plasmids in general, and A-type 2μ plasmids, in particular. However, this effect can be confounded by the phylogenetic relatedness of these strains.