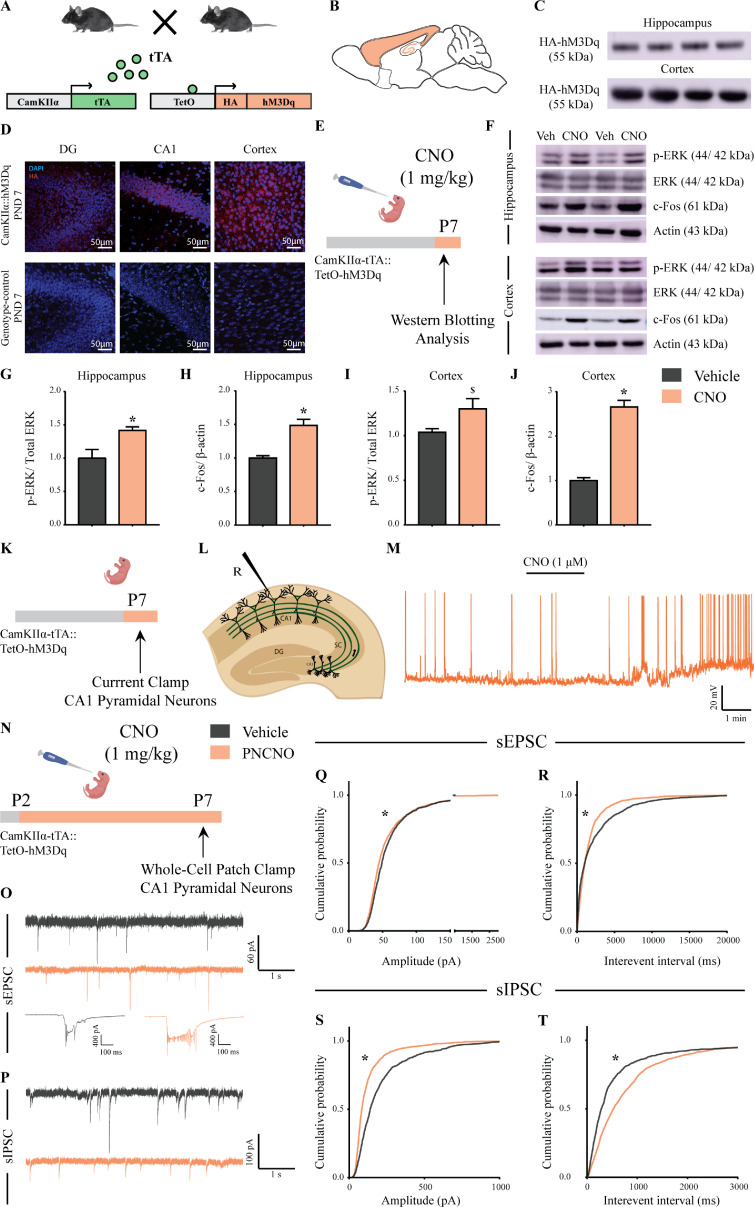
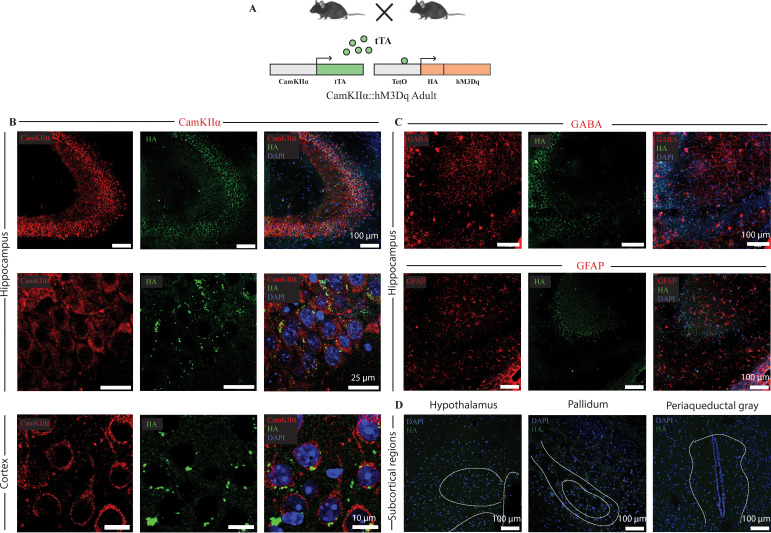
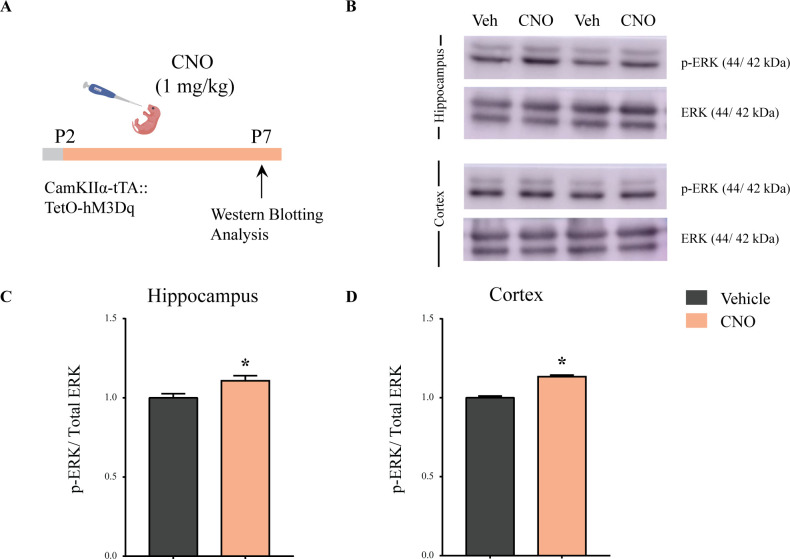
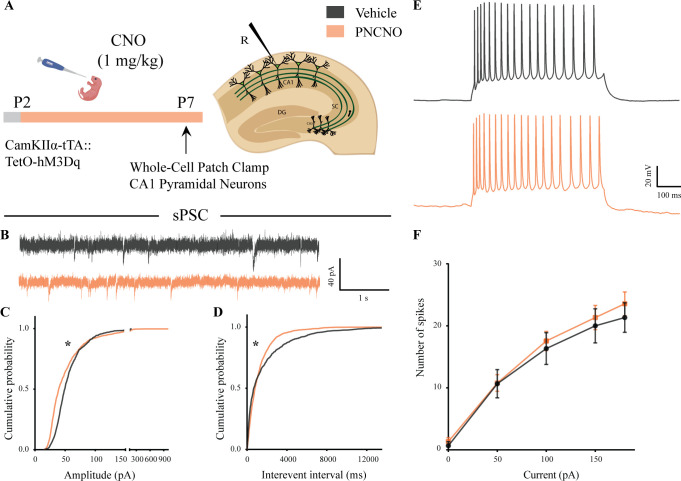
Figure 1. Selective expression and activation of hM3Dq DREADD in CamKIIα-positive forebrain excitatory neurons in CamKIIα-tTA::TetO-hM3Dq bigenic mice during the postnatal window.
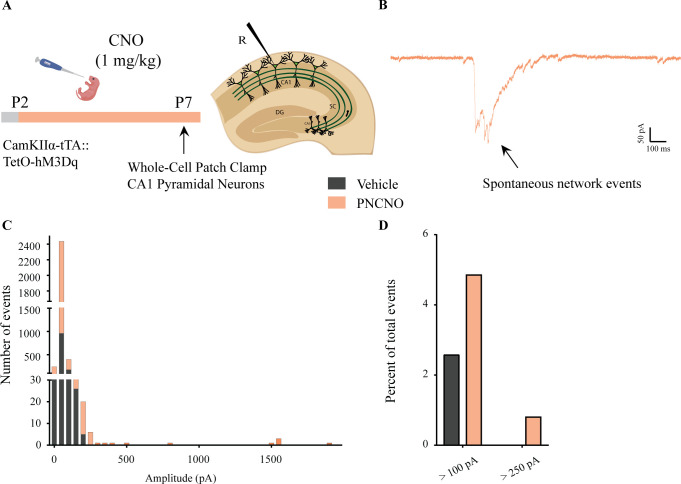
(A) Shown is a schematic of the experimental strategy for the generation of the bigenic CamKIIα-tTA::TetO-hM3Dq mouse line to selectively drive the expression of the hM3Dq DREADD in CamKIIα-positive forebrain excitatory neurons. tTA – tetracycline transactivator. (B) Shown is a schematic sagittal view of the mouse brain indicating the region of hM3Dq DREADD expression. (C) Western blots indicate expression of the HA-tag in the hippocampus and the cortex confirming the presence of HA-tagged hM3Dq DREADD (n = 4). (D) Shown are representative confocal images indicating expression of hM3Dq DREADD in the DG, CA1, and cortex as identified by HA immunofluorescence, which was not observed in the genotype-control mice (n = 3 per group). (E) Shown is the experimental paradigm to assess activity-related signaling signatures following acute CNO-mediated hM3Dq DREADD activation of CamKIIα-positive forebrain excitatory neurons at P7. The mice were fed a single dose of either CNO (1 mg/kg) or vehicle and sacrificed 15 min later for western blotting analysis (n = 4 per group). (F) Representative western blots indicate the expression of the neuronal activity-related proteins, p-ERK and c-Fos in the hippocampus and cortex of CNO and vehicle-treated CamKIIα-tTA::TetO-hM3Dq bigenic mouse pups. Densitometric quantification revealed a significant CNO-mediated, hM3Dq DREADD activation evoked increase in p-ERK/ERK (G) and c-Fos (H) expression in the hippocampi of CNO-treated pups as compared to the vehicle-treated controls (n = 4 per group). In the cortex, hM3Dq DREADD activation resulted in a trend toward an increase in p-ERK/ERK (I) and a significant increase in c-Fos (J) protein levels in the CNO-treated pups. Results are expressed as the mean ± S.E.M. *p<0.05, $p=0.07 as compared to vehicle-treated controls using the two-tailed, unpaired Student’s t-test. (K–L) Shown is a schematic of the experimental paradigm for whole-cell patch clamp recording from the somata of CA1 pyramidal neurons at P7 in acute hippocampal slices derived from drug-naïve, bigenic CamKIIα-tTA::TetO-hM3Dq mouse pups. R – Recording electrode. (M) Bath application of CNO (1 μM) to acute hippocampal slices resulted in hM3Dq DREADD activation mediated robust spiking activity of CA1 pyramidal neurons (n = 3 cells). (N) Experimental paradigm to assess the effects of chronic CNO-mediated hM3Dq DREADD activation of CamKIIα-positive forebrain excitatory neurons using whole-cell patch clamp recording. CamKIIα-tTA::TetO-hM3Dq bigenic mouse pups were fed either CNO (1 mg/kg) or vehicle from P2 to P7 followed by recording of sEPSCs and sIPSCs. (O) Shown are representative sEPSC traces of vehicle and PNCNO-treated mice at P7. Top traces: examples of small amplitude events. Bottom traces: examples of large-amplitude events. (P) Shown are representative sIPSC traces of vehicle and PNCNO-treated mice at P7. (Q) PNCNO-treated mice showed significantly altered cumulative probability of sEPSC amplitude with a small decrease at lower amplitudes (<100 pA) and a significant increase in large-amplitude events characterized by a long-tail as compared to vehicle-treated controls. (R) PNCNO-treated mice showed a significant decline in the cumulative probability of sEPSC interevent intervals as compared to vehicle-treated controls (n = 7 cells for vehicle; n = 10 cells for PNCNO). PNCNO-treated mice showed a significant decrease in sIPSC amplitude (S), and a concomitant increase in sIPSC interevent intervals (T) as compared to vehicle-treated controls (n = 6 cells for vehicle; n = 8 cells for PNCNO). Results are expressed as cumulative probabilities. *p<0.001 as compared to PNCNO-treated group using Kolmogorov-Smirnov two-sample comparison.