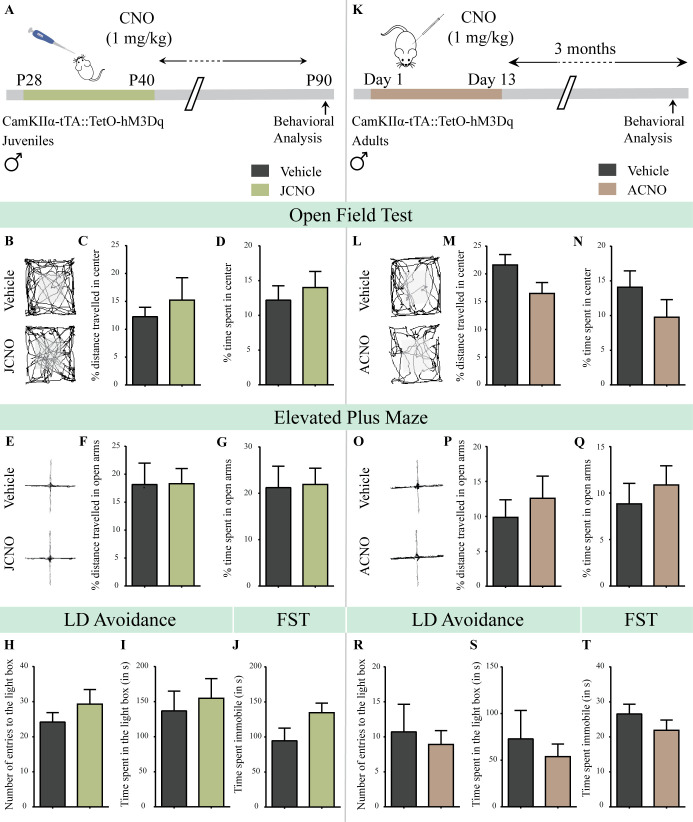
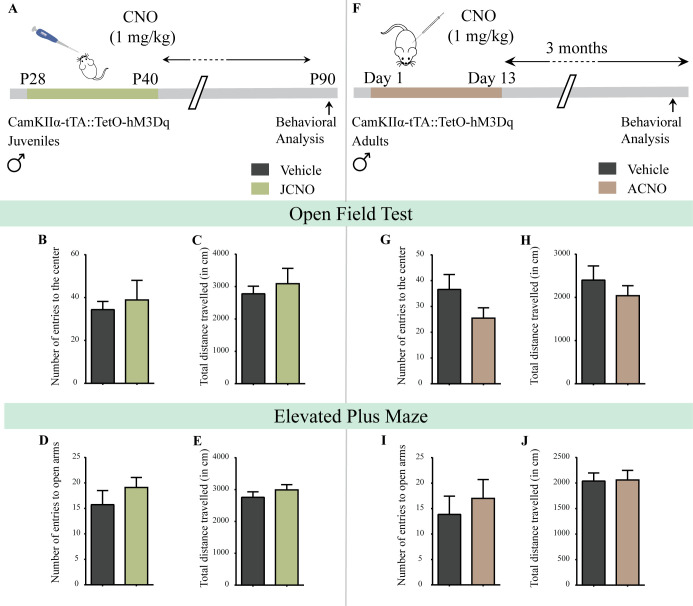
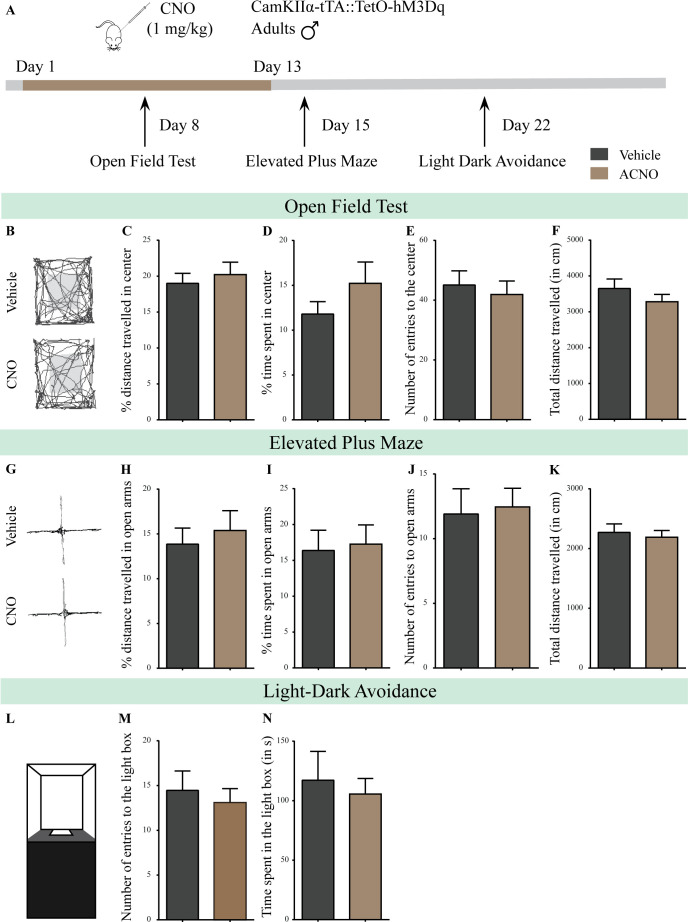
Figure 3. Chronic chemogenetic activation of CamKIIα-positive forebrain excitatory neurons during the juvenile window or in adulthood does not evoke any long-lasting changes in anxiety- and despair-like behavior in male mice.
(A) Shown is a schematic of the experimental paradigm to induce chronic CNO-mediated hM3Dq DREADD activation in CamKIIα-positive forebrain excitatory neurons using bigenic CamKIIα-tTA::TetO-hM3Dq juvenile male mice that were fed CNO (JCNO; 1 mg/kg) or vehicle from P28-P40 and then left undisturbed till 3 months of age prior to behavioral analysis. (B) Shown are representative tracks of vehicle or JCNO-treated CamKIIα-tTA::TetO-hM3Dq bigenic adult male mice in the open field test (OFT). A history of chronic juvenile hM3Dq DREADD activation of CamKIIα-positive forebrain excitatory neurons does not evoke any persistent change in anxiety-like behavior on the OFT in adulthood, with no difference observed in the percent distance traveled in the center (C) and the percent time spent in the center (D) of the OFT arena in JCNO-treated mice as compared to vehicle-treated controls (n = 11 for vehicle; n = 8 for JCNO). (E) Shown are representative tracks of vehicle or JCNO-treated CamKIIα-tTA::TetO-hM3Dq bigenic adult mice on the elevated plus maze (EPM). Adult mice with chronic juvenile hM3Dq DREADD activation of CamKIIα-positive forebrain excitatory neurons did not exhibit any persistent changes in anxiety-like behavior on the EPM, with no change observed for the percent distance traveled (F) and the percent time spent (G) in the open arms of the EPM arena across treatment groups (n = 11 for vehicle; n = 10 for JCNO). Anxiety-like behavior was also assessed on the light-dark avoidance test and no significant alterations were seen in the number of entries to the light box (H) or the time spent in the light box (I) across treatment groups (n = 11 for vehicle; n = 10 for JCNO). A history of chronic juvenile hM3Dq DREADD activation of CamKIIα-positive forebrain excitatory neurons did not evoke any persistent change in despair-like behavior in the forced swim test (FST), with no difference observed in the time spent immobile (J) in JCNO-treated mice as compared to vehicle-treated controls (n = 11 per group). (K) Shown is a schematic of the experimental paradigm to induce chronic CNO-mediated hM3Dq DREADD activation in CamKIIα-positive forebrain excitatory neurons using bigenic CamKIIα-tTA::TetO-hM3Dq adult male mice (3–4 months of age) that received CNO (ACNO; 1 mg/kg) or vehicle via intraperitoneal administration (once daily for thirteen days) and were left undisturbed for 3 months prior to behavioral analysis. (L) Shown are representative tracks of vehicle or ACNO-treated CamKIIα-tTA::TetO-hM3Dq bigenic adult male mice in the open field test (OFT). A history of chronic hM3Dq DREADD activation of CamKIIα-positive forebrain excitatory neurons in adulthood does not evoke any persistent change in anxiety-like behavior on the OFT, with no difference observed in the percent distance traveled in the center (M) and the percent time spent in the center (N) of the OFT arena in ACNO-treated mice as compared to vehicle-treated controls (n = 7 for vehicle; n = 11 for ACNO). (O) Shown are representative tracks of vehicle or ACNO-treated CamKIIα-tTA::TetO-hM3Dq bigenic adult mice on the elevated plus maze (EPM). Chronic hM3Dq DREADD activation of CamKIIα-positive forebrain excitatory neurons in adulthood did not evoke any persistent changes in anxiety-like behavior on the EPM, with no change observed for the percent distance traveled (P) and the percent time spent (Q) in the open arms of the EPM arena across treatment groups (n = 7 for vehicle; n = 11 for ACNO). Anxiety-like behavior was also studied on the light-dark avoidance test and no change was observed in the number of entries to the light box (R) or the time spent in the light box (S) across treatment groups (n = 7 for vehicle; n = 11 for ACNO). Chronic hM3Dq DREADD activation of CamKIIα-positive forebrain excitatory neurons in adulthood did not evoke any persistent change in despair-like behavior in the forced swim test (FST), with no difference observed in the time spent immobile (T) in ACNO-treated mice as compared to vehicle-treated controls (n = 10 for vehicle; n = 9 for ACNO). Results are expressed as the mean ± S.E.M.