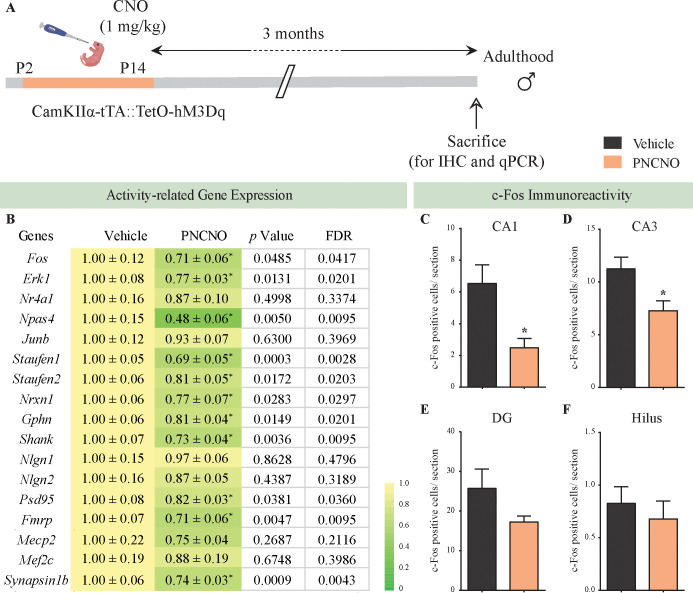
Figure 6. Chronic chemogenetic activation of CamKIIα-positive forebrain excitatory neurons during the early postnatal window results in a long-lasting decline in neuronal activity-related gene expression, and c-Fos immunopositive cell numbers, in the adult hippocampus.
(A) Shown is a schematic of the experimental paradigm to induce chronic CNO-mediated hM3Dq DREADD activation in CamKIIα-positive forebrain excitatory neurons using bigenic CamKIIα-tTA::TetO-hM3Dq mouse pups that were fed CNO (PNCNO; 1 mg/kg) or vehicle from P2 to P14 and then left undisturbed for 3 months prior to qPCR analysis for neuronal activity-related gene expression and c-Fos immunohistochemistry performed in adulthood on male mice. (B) Shown are normalized gene expression levels for specific neuronal activity-related genes in PNCNO-treated mice represented as fold-change of their vehicle-treated controls (n = 11 per group). Heat maps indicate the extent of gene regulation. *p<0.05 as compared to vehicle-treated controls using the two-tailed, unpaired Student’s t-test. Also shown are false-discovery rate (FDR) corrected p values. Cell counting analysis for c-Fos immunopositive cells within the hippocampus, indicated a significant reduction in the number of c-Fos positive cells/section within the CA1 (C) and CA3 (D) hippocampal subfields in PNCNO-treated mice as compared to vehicle-treated controls (n = 10 for vehicle; n = 12 for PNCNO). c-Fos immunopositive cell numbers were unaltered in the DG (E) and the hilar subfield (F) of the hippocampus in PNCNO-treated mice as compared to vehicle-treated controls. Results are expressed as the mean ± S.E.M. *p<0.05 as compared to vehicle-treated controls using the two-tailed unpaired Student’s t-test.