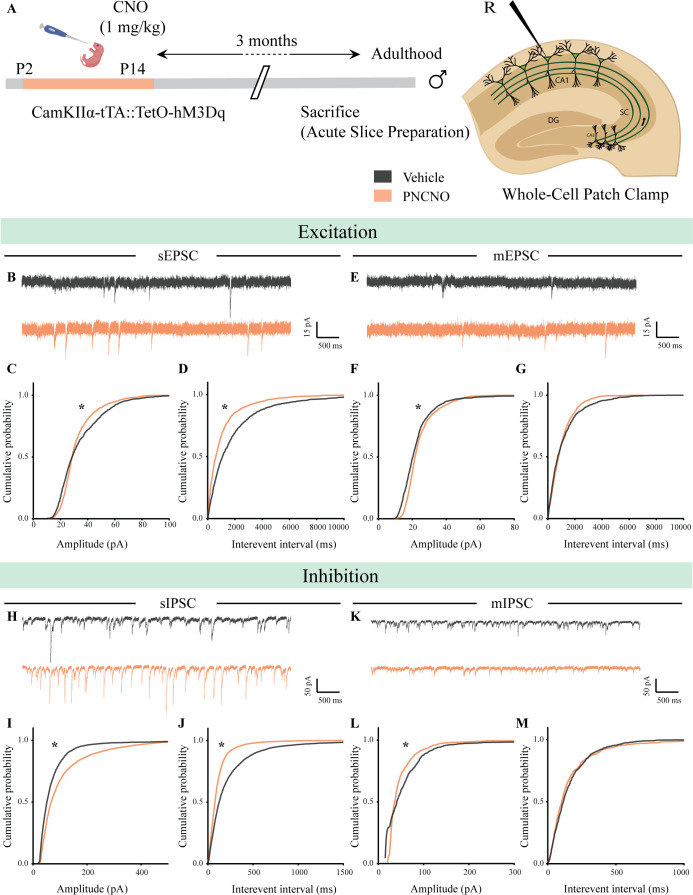
Figure 7. Chronic chemogenetic activation of CamKIIα-positive forebrain excitatory neurons during the early postnatal window alters excitatory and inhibitory spontaneous currents in the hippocampi of adult male mice.
(A) Shown is a schematic of the experimental paradigm to induce chronic CNO-mediated hM3Dq DREADD activation in CamKIIα-positive forebrain excitatory neurons using bigenic CamKIIα-tTA::TetO-hM3Dq mouse pups that were fed CNO (PNCNO; 1 mg/kg) or vehicle from P2 to P14 and then left undisturbed for 3 months prior to electrophysiological analysis, in acute hippocampal slices derived from adult male mice. Whole-cell patch clamp was performed to record sEPSCs/mEPSCs and sIPSCs/mIPSCs in the somata of CA1 pyramidal neurons. R – Recording electrode. (B) Shown are representative sEPSC traces of CA1 pyramidal neurons from vehicle and PNCNO-treated mice. (C) PNCNO-treated mice showed significantly altered cumulative probability of sEPSC amplitude with a small increase at lower amplitudes (<30 pA) and a significant decline in large-amplitude events as compared to vehicle-treated controls. (D) PNCNO-treated mice showed a significant decline in the cumulative probability of sEPSC interevent intervals as compared to vehicle-treated controls (n = 8 cells for vehicle; n = 5 cells for PNCNO). (E) Shown are representative mEPSC traces of CA1 pyramidal neurons from vehicle and PNCNO-treated mice. (F) PNCNO-treated mice showed significantly enhanced cumulative probability of sEPSC amplitude as compared to vehicle-treated controls. (G) No significant change was observed in the cumulative probability of sEPSC interevent intervals in PNCNO-treated mice as compared to vehicle-treated controls (n = 5 cells for vehicle; n = 6 cells for PNCNO). (H) Shown are representative sIPSC traces of CA1 pyramidal neurons from vehicle and PNCNO-treated mice. PNCNO-treated mice showed a significant increase in the cumulative probability of sIPSC amplitude (I), along with a significant decline in the cumulative probability of sIPSC interevent intervals (J) as compared to vehicle-treated controls (n = 8 cells for vehicle; n = 7 cells for PNCNO). (K) Shown are representative mIPSC traces of CA1 pyramidal neurons from vehicle and PNCNO-treated mice. (L) PNCNO-treated mice showed a significant decline in the cumulative probability of mIPSC amplitude as compared to vehicle-treated controls (n = 6 cells for vehicle; n = 7 cells for PNCNO). (M) No significant change was observed in the cumulative probability of mIPSC interevent intervals across treatment groups. Results are expressed as cumulative probabilities. *p<0.001 as compared to PNCNO-treated group using the Kolmogorov-Smirnov two-sample comparison.