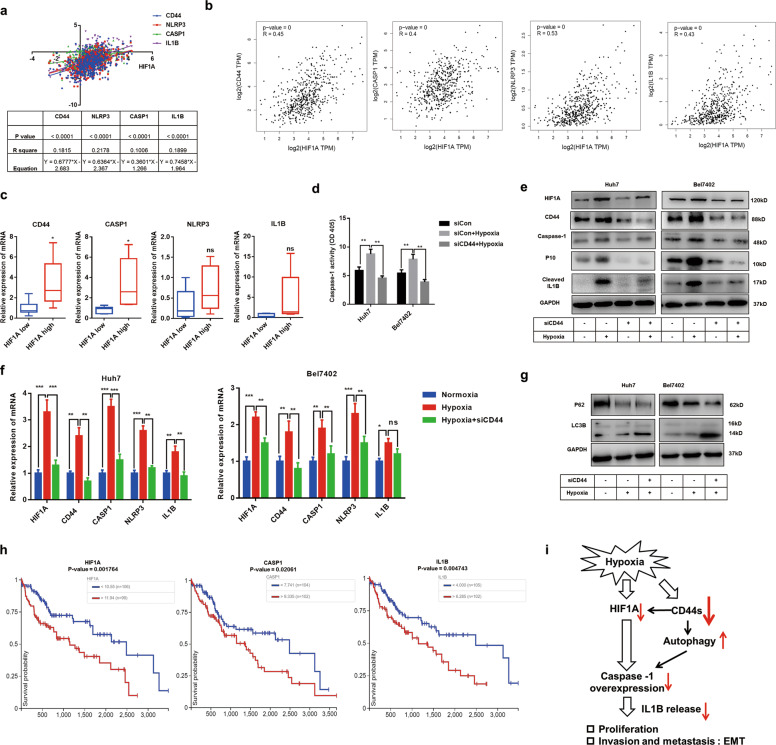
Fig. 6. Targeting CD44s represses hypoxia-induced caspase-1/IL1B via decreasing HIF1A and increasing autophagy.
a A positive correlation between HIF1A, CD44, and genes involved in the caspase-1/IL1B pathway. Relevant data were extracted from UCSC Xena for correlation analysis. b A positive correlation between HIF1A, CD44, and genes involved in the caspase-1/IL1B pathway. Relevant data were extracted from GEPIA for correlation analysis. c mRNA expression of CD44 and genes involved in the caspase-1/IL1B pathway in HCC tissues with high or low HIF1A expression. n = 50. d Caspase-1 activity analyses were performed in three groups, and results suggested that siCD44 treatment inhibited hypoxia-induced caspase-1 capacity. e, f Q-PCR and immunoblot analysis both indicated the downregulation of CD44s inhibits hypoxia-induced caspase-1 expression via reducing HIF1A. g Immunoblot analysis suggested targeting CD44s further promoted autophagy under hypoxia condition. h HIF1A, CASP1, and IL1B expression are significantly related to OS in HCC. Data were extracted from the UCSC Xena database. i The schematic outline of this study. In normoxia conditions, CD44s deficiency promotes p62-mediated autophagic degradation of caspase-1, which results in suppression of the caspase-1/IL1B pathway. In hypoxia conditions, targeting CD44s inhibits hypoxia-induced activation of caspase-1/IL1B through decreasing HIF1A and increasing autophagy. Data are means ± SEM from 3 independent experiments, * means p < 0.05, ** means p < 0.01, *** means p < 0.001 by unpaired student t test.