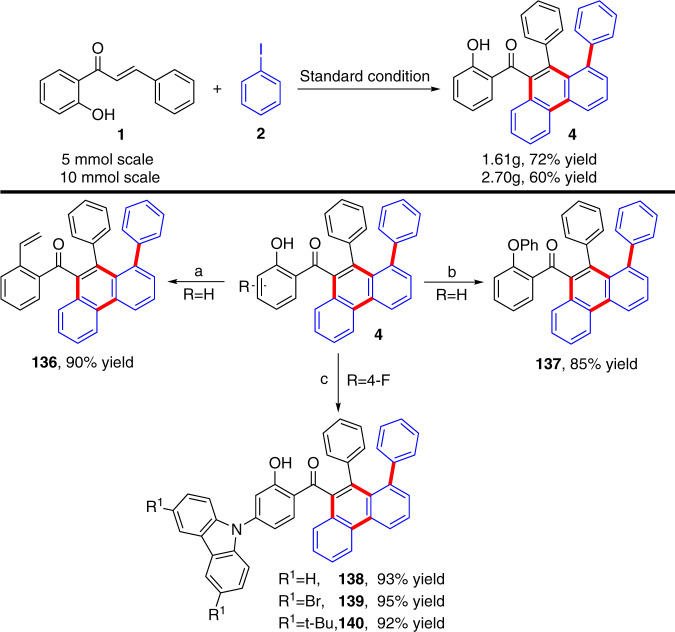
Fig. 5. Further transformations.
a 1) Et3N (2 eq), Tf2O (2 eq), DCM, r.t. 2) Pd(PPh3)4 (5 mol%), LiCl (2 eq), Bu3SnC2H3 (1.2 eq), DMF, Ar, 70 °C, 12 h. b PhI (1.5 eq), CuI (10 mol%), N,N-dimethylglycine (22 mol%), K3PO4 (2 eq), DMSO, Ar, 100 °C, 12 h. c Carbazole derivatives (3 eq), t-BuOK (3 eq), DMF, Ar, 150 °C, 72 h. All yield are isolated products.