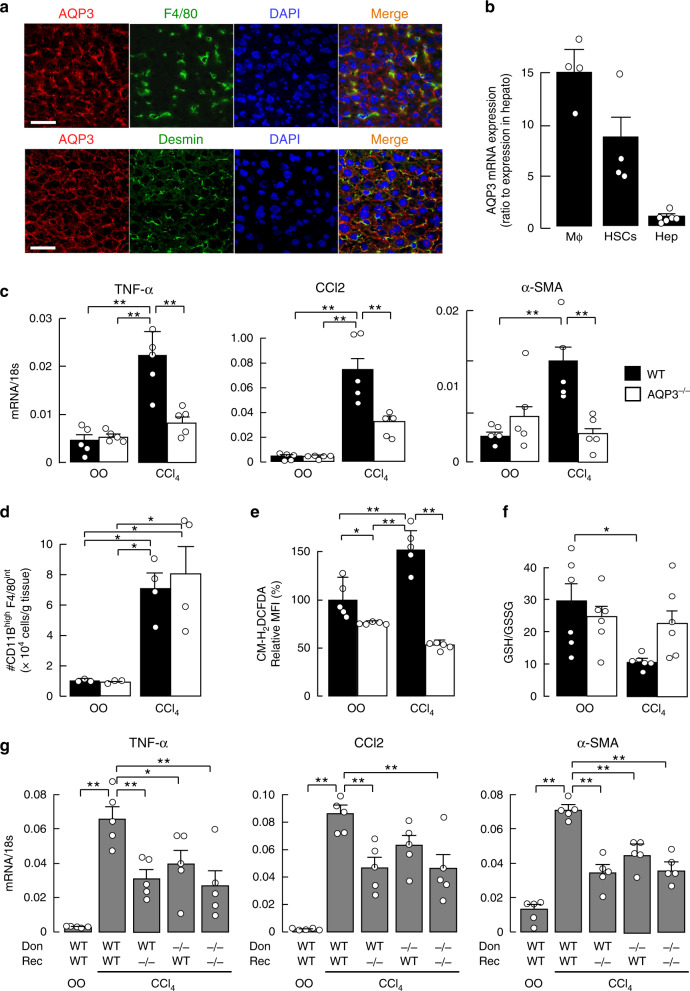
Fig. 1. Reduced CCl4-induced acute liver injury in AQP3−/− mice.
a AQP3 immunofluorescence in mouse liver. Immunostaining with anti-AQP3 (Cy3, red), and anti-F4/80 (FITC, green, upper) or anti-desmin (FITC, green, lower). Bar, 100 µm. b AQP3 mRNA expression in hepatic macrophages (Mϕ), hepatic stellate cells (HSCs), and hepatocytes (Hep) from WT liver determined by real-time RT-PCR (mean ± SE, n = 6 for hepatocytes, n = 4 for macrophages and HSCs biologically independent samples). Data are expressed as the ratio to 18 s RNA. c–f CCl4 (1 ml/kg) or vehicle olive oil (OO) was injected intraperitoneally, and livers were excised at 24 h. c Expression of mRNAs encoding TNF-α, CCl2, and α-SMA by real-time RT-PCR (mean ± SE, n = 5 mice/group, **p < 0.01). Data are expressed as the ratio to 18 s RNA. d Dispersed liver cells were stained with anti-F4/80 and anti-CD11B, and analyzed by FACS, as shown in Supplementary Fig. 1d. Cell number of CD11Bhigh F4/80intermediate hepatic macrophages (mean ± SE, n = 3 for OO, n = 4 for CCl4 mice/group, *p < 0.05). e Mean fluorescence intensity (MFI) of CM-H2DCFDA by FACS analysis in hepatocytes (mean ± SE, n = 5 biologically independent samples *p < 0.05, **p < 0.01). f Ratio of GSH to GSSG in liver homogenate (mean ± SE, n = 6 mice/group, *p < 0.05). Statistical analysis for (c)–(f) was performed by two-way ANOVA with Tukey’s multiple comparisons test. g Mice (WT or AQP3−/−, age 8–10 weeks) were gamma-irradiated (900 rad) and injected intravenously with bone marrow from WT or AQP3−/− mice. The study was performed at 60 days after bone marrow transfer (mean ± SE, n = 5 mice/group, *p < 0.05, **p < 0.01 by one-way ANOVA with Dunnett’s multiple comparisons test). Source data, including exact p values, are provided as a Source data file.