Table 1.
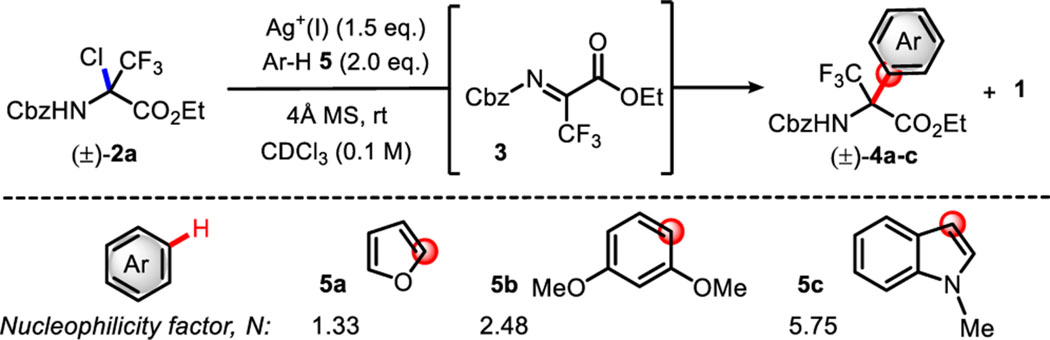 | |||||
|---|---|---|---|---|---|
| conversion ratio (2:3:1:4)b | product yieldb,c (%) |
||||
| entry | arene | silver source | 1 h | 4 h | 1 day |
| 1d | - | Ag2CO3 | 66:34:0:0 | 25:40:35:0 | >95 (1) |
| 2 | - | Ag2CO3 | 61:36:3:0 | 39:59:2:0 | 98 (3) |
| 3 | 5a | Ag2CO3 | 53:45:2:0 | 17:78:5:0 | >95 (3) |
| 4 | 5b | Ag2CO3 | 70:2:2:0 | 26:70:1:0 | >98 (3)e |
| 5 | 5c | Ag2CO3 | 54:9:5:32 | 36:9:5:50 | 98 (4c) |
| 6 | 5b | AgOTf | 42:0:17:20 | 0:0:16:15 | 36 (4b) |
| 7f | 5b | AgOTf | 0:0:32:42 | 0:0:52:33 | 54 (4b) |
| 8f,g | 5b | AgOTf | 0:0:40:39 | 0:0:24:53 | 71 (4b) |
Reactions were carried out under argon with 2 (0.10 mmol) with arenes 5a-c(2.0 equiv), silver reagent [1.5 equiv in Ag(I)], and 30 mg of 4 Å molecular sieves in CDCl3 (2.0 mL).
NMR ratios and yields determined for the crude reaction mixture by 19F NMR with C6F6 as the internal standard.
NMR yields determined for the crude reaction mixture by 1H NMR with mesitylene as the internal standard.
Reaction carried out without 4 Å molecular sieves.
The reaction was also carried out at higher temperatures (up to 60 °C), and the formation of 4b was not observed.
Reaction carried out in anhydrous Et2O.
Reaction carried out at 0.3 M.
