Figure 4.
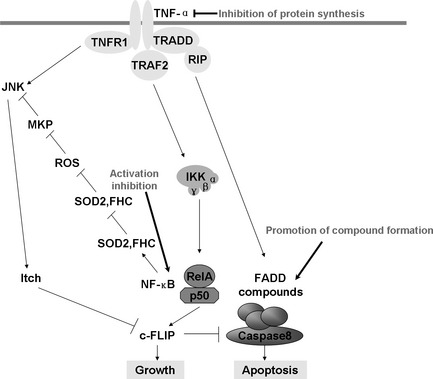
Activation of TNF‐α pathway and possible strategies to prevent resistance to radiation through inhibiting the activity of relative elements. Although TNF can bind two receptors, TNF‐R1 (TNF receptor type 1) and TNF‐R2 (TNF receptor type 2); most information regarding TNF signaling is derived from TNF‐R1. Upon contact with their ligands, TNF receptors form trimers and cause a conformational change, leading to the dissociation of the inhibitory protein SODD from the intracellular death domain and leading the adaptor protein TRADD to be binded. Following TRADD binding, three pathways can be initiated. First, TRADD recruits TRAF2 and RIP, then TRAF2, in turn, recruits the multicomponent protein kinase IKK. IKK phosphorylates an inhibitory protein, IκBα, and releases NF‐κB, which can translocate to the nucleus and mediate the transcription of a vast array of proteins involved in cell survival and proliferation, inflammatory response and anti‐apoptotic factors. Second, TNFR induces a strong activation of the stress‐related JNK group, evokes moderate response of the p38‐MAPK, and is responsible for minimal activation of the classical ERK. Of the three major TNF‐α cascades, TNFR is also involved in death signaling. TRADD binds FADD, which then recruits the cysteine protease caspase‐8. A high concentration of caspase‐8 induces its autoproteolytic activation and subsequent cleaves of effector caspases, leading to cell apoptosis. Possible strategies to prevent acquired resistance include combination therapy against TNF‐α protein synthesis in the membrance and NF‐κB activation or promoting FADD/Caspase 8 compound formation.
