Table 4.
Free Primary and Secondary Cyclopropylmethylamine Scope for γ-C(sp3)−H Carbonylation and Olefinationa,b
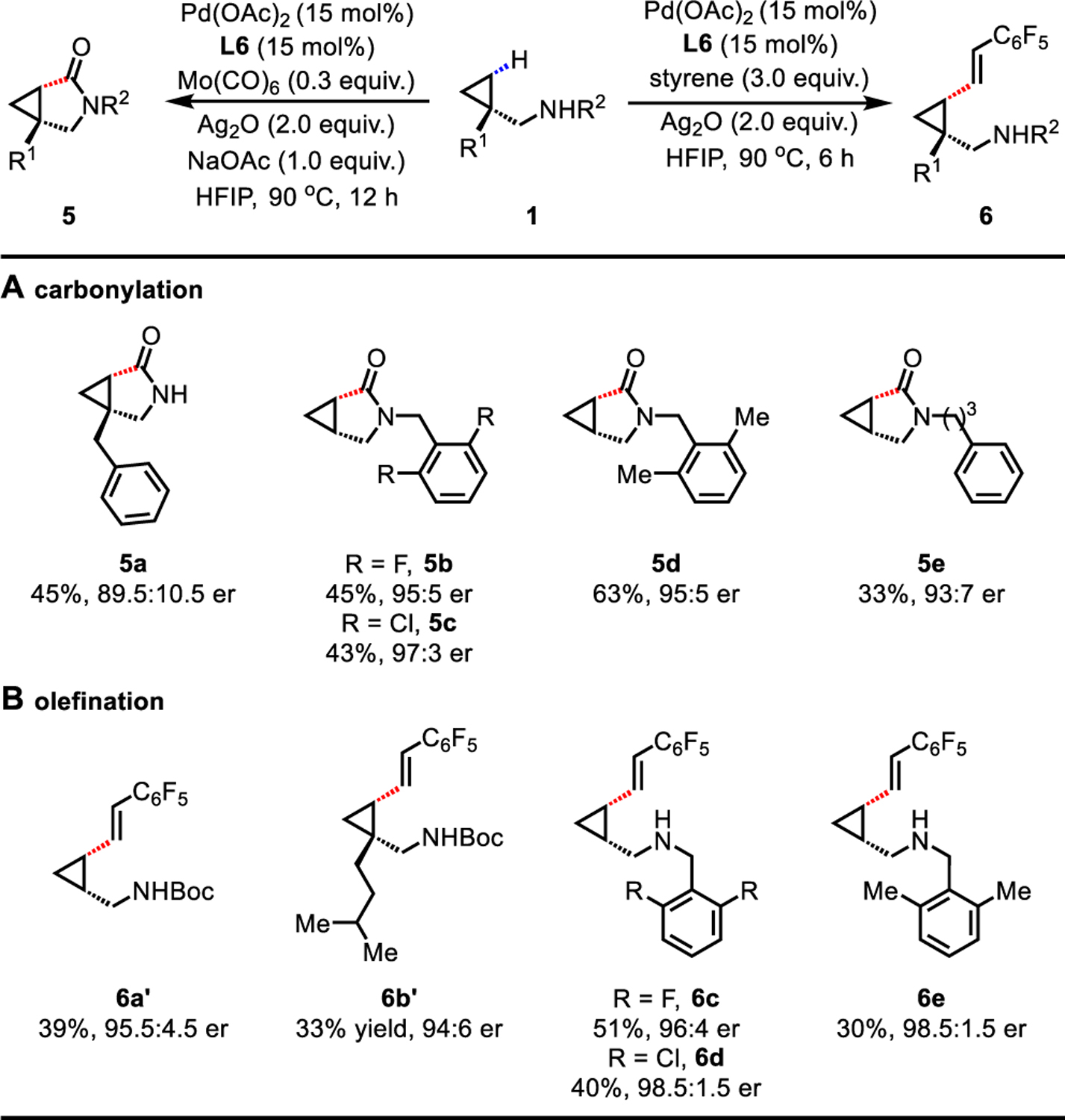 |
Conditions for carbonylation: 1 (0.1 mmol), Pd(OAc)2 (15 mol%), L6 (15 mol%), Mo(CO)6 (0.3 equiv.), Ag2O (2.0 equiv.), NaOAc (1.0 equiv.), HFIP (0.1 mL), 90 °C, 12 h. Conditions for olefination: 1 (0.1 mmol), Pd(OAc)2 (15 mol%), L6 (15 mol%), pentafluorostyrene (3.0 equiv.), Ag2O (2.0 equiv.), HFIP (0.1 mL), 90 °C, 6 h.
Isolated yields for secondary amines or isolated yields of the corresponding Boc-protected amines for primary amines. The er values were determined on the SFC system using commercially available chiral columns.
