Abstract
Background:
Patients with heart failure (HF) have multiple co-existing comorbidities. The temporal trends in the burden of comorbidities and associated risk of mortality among patients with HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF) are not well-established.
Methods:
HF related hospitalizations were sampled by stratified design from four US areas in 2005 to 2014 by the community surveillance component of the ARIC (Atherosclerosis Risk in Communities) study. Acute decompensated HF was classified by standardized physician review and a previously validated algorithm. An ejection fraction <50% was considered HFrEF. A total of 15 co-morbidities were abstracted from the medical record. Mortality outcomes were ascertained for up to 1-year post-admission, by linking hospital records with death files.
Results:
A total of 5,460 hospitalizations (24,937 weighted hospitalizations) classified as acute decompensated HF had available ejection fraction data (53% female, 68% white, 53% HFrEF, 47% HFpEF). The average number of comorbidities was higher for patients with HFpEF vs. HFrEF, both for women (5.53 vs. 4.94, P<0.0001) and men (5.20 vs. 4.82, P<0.0001). There was a significant temporal increase in the overall burden of co-morbidities, both for patients with HFpEF (Women: 5.17 in 2005–2009 to 5.87 in 2010–2013; Men: 4.94 in 2005–2009 and 5.45 in 2010–2013) and HFrEF (Women: 4.78 in 2005–2009 to 5.14 in 2010–2013; Men: 4.62 in 2005–2009 and 5.06 in 2010–2013, P-trend<0.0001 for all). Higher comorbidity burden was significantly associated with higher adjusted risk of 1-year mortality, with a stronger association noted for HFpEF [HR (95% CI) per 1-higher co-morbidity:1.19(1.14–1.25) vs. HFrEF [HR (95%CI): 1.10(1.05–1.14); P-interaction by HF type = 0.02]. The associated mortality risk per 1-higher co-morbidity also increased significantly over time for patients with HFpEF as well HFrEF (P-for interaction with time =0.002 and 0.02 respectively)
Conclusion:
The burden of comorbidities among hospitalized patients with acute decompensated HFpEF and HFrEF has increased over time, as has its associated mortality risk. Higher burden of comorbidities is associated with higher risk of mortality, with a stronger association noted among patients with HFpEF vs. HFrEF.
Keywords: Co-morbidities, Heart failure with preserved ejection fraction, Heart failure with reduced ejection fraction, Mortality
Introduction
Heart failure (HF) is increasing in prevalence, is associated with high morbidity and mortality burden, and contributes substantially to the health care cost in the United States and worldwide.1–3 Patients with HF have multiple co-existing conditions that contribute to the impairment in functional status and excess risk of adverse clinical outcomes.4–6 Recent studies have reported an increasing prevalence of both cardiovascular and non-cardiovascular comorbidities among patients hospitalized with HF.7, 8 Over the past few decades, two distinct phenotypes of HF have been recognized, HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF).9 While the incidence of HFrEF has declined over the past few years, that of HFpEF continues to increase, particularly among older hospitalized individuals.10–12 Furthermore, compared with HFrEF, HFpEF is more often burdened by excessive comorbidities.13, 14 Thus, it is unclear whether the temporal trends in comorbidities reflect increasing medical complexity of patients hospitalized with acute HF, or an increasing predominance of HFpEF. Furthermore, the temporal changes in the prognostic implications associated with higher co-morbidity burden in HFpEF and HFrEF is not well known. This represents an important knowledge gap as many comorbid conditions may be modifiable, presenting actionable areas for intervention to improve outcomes in this high-risk patient population.
Accordingly, in this study, we examined the temporal trends in the burden of different co-morbidities in patients hospitalized with acute decompensated HFpEF and HFrEF using community surveillance data captured by the Atherosclerosis Risk in Communities Study (ARIC) study from 2005–2014. We also evaluated the temporal trends in the association between the overall comorbidity burden and length of stay and risk of mortality among these patients.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
The ARIC Study Community Surveillance
Since 2005, the ARIC study has conducted population-based retrospective surveillance of hospitalized events in Forsyth County, North Carolina; Washington County, Maryland; Jackson, Mississippi; and 8 northwest suburbs of Minneapolis, Minnesota. Surveillance eligibility is restricted to residents 55 years of age or older, with a hospitalization spanning at least one day and, for the purposes of our analysis, a discharge date between January 1, 2005 – December 31, 2014. Hospitalizations with any discharge codes for congestive heart failure, rheumatic heart disease, hypertensive heart disease, acute cor pulmonale, chronic pulmonary heart disease, cardiomyopathies, acute edema of lung, or dyspnea were randomly sampled, using pre-specified sampling fractions within strata of ARIC communities, ICD-9 code (428.x or all other eligible codes), age (55–74, 75–84, or ≥85), sex, and race (black or white).12, 15 Surveillance activities were approved by local institutional review boards from the 4 ARIC communities and all study data and materials are publicly available. Patient consent was not required for surveillance because all personal identifiers were redacted.
Event adjudication
Hospitalized medical records indicating signs or symptoms of heart failure were fully abstracted and reviewed by ARIC physicians, as previously described.15 Using standardized criteria, hospitalizations were classified as definite acute decompensated HF, probable acute decompensated HF, stable chronic heart failure, not heart failure, or unclassifiable; based on diagnostic reports from the hospital record, physician notes, and discharge summaries. Acute decompensated HF was differentiated from stable, chronic heart failure by evidence of new onset or worsening signs or symptoms.15
Clinical data abstraction
Demographic and clinical data were abstracted from the medical record by certified study personnel following a standardized protocol. The following clinical variables were routinely abstracted from the medical record at all hospitals and throughout the study period: coronary artery disease, peripheral artery disease, hypertension, pulmonary hypertension, atrial fibrillation, stroke/transient ischemic attack (TIA), valvular heart disease, myocardial infarction, body mass index, diabetes mellitus, serum creatinine, chronic obstructive pulmonary disease (COPD), sleep apnea, depression, anemia, and thyroid disease. Although myocardial infarction was included in the definition for coronary artery disease, it was also recorded as a separate clinical variable. Obesity was defined by abstracted height at admission and weight at hospital discharge (defining obesity by a body mass index > 30 kg/m2). Chronic kidney disease (CKD) was defined by receipt of hemodialysis or an estimated glomerular filtration rate <45 mL/min/1.73 m2, using serum creatinine values and the CKD Epidemiology Collaboration formula. However, serum creatinine was not abstracted in 2014, limiting our analysis of CKD to 2005 – 2013. Anemia was defined by an abstracted hemoglobin value < 11 g/dL. To minimize the effect of hemodilution on laboratory values, we used the last value recorded over the course of hospitalization for serum creatinine and anemia.
Heart failure type
Heart failure type was determined by the abstracted ejection fraction (EF), from either inpatient diagnostic tests, or when absent, pre-admission imaging studies, as previously reported.12 Heart failure with reduced ejection fraction (HFrEF) was identified by reported EF < 50% and heart failure with preserved ejection (HFpEF) was identified by EF ≥50%.
Procedures and Therapies
Invasive cardiac procedures (e.g., coronary angiography, coronary artery bypass graft, percutaneous coronary intervention) were abstracted from the medical record if performed during the acute decompensated HF hospitalization or within 2 years prior to hospitalization. Heart failure medications (e.g., beta blockers, angiotensin converting enzyme inhibitors and angiotensin receptor blockers [ACEi / ARBs] were abstracted from the medical record if administered prior to or during the hospital stay).
Outcomes
Length of stay was calculated by subtracting the admission date from the discharge date, excluding transfers to and from another hospital. Mortality outcomes were ascertained for up to 1-year post-admission, by linking hospital records with death files.
Statistical Analysis
Statistical tests and models accounted for the stratified sampling design and were weighted by the inverse of the sampling probability (13). Continuous variables were assessed for normality and compared using the difference in least square means from weighted linear regression. Categorical variables were compared using Rao-Scott χ2 tests. Baseline characteristics of the study participants were stratified by sex and HF type and presented within half-decades. Temporal trends in comorbidities were visualized by plotting annual prevalence across 2005–2014, stratified by sex and by HF type, with trend lines fit by 2nd order polynomials. Significance of temporal trends was derived from logistic regression, constructing separate models for each comorbidity and regressing on year of admission. Differences in temporal trends were compared between patients with HFrEF vs. HFpEF by testing the multiplicative interaction of HF type and year of admission. Comorbidity burden (number of comorbidities per patient) was quantified for patients with complete abstractions for all 15 comorbidities and was limited to hospitalizations from 2005–2013 because serum creatinine was not abstracted in 2014. A total of 15, rather than 16 co-morbidities was considered to assess overall co-morbidity burden because myocardial infarction was included in the definition of coronary artery disease. Associations between individual and total number of comorbidities with all-cause mortality at 28-days and 1 year of hospitalization were analyzed using Cox regression, with follow up time administratively censored at the end of each study interval. Models were stratified by sex, HF type, and half-decade and adjusted for age, race, sex, year of admission and hospital of admission. Modification of mortality outcomes by HF type was tested by the multiplicative interaction between HF type and number of comorbidities. As a sensitivity analysis, we also examined the 1-year mortality associated with total number of “primary” risk factors for HF (hypertension, obesity, diabetes, chronic kidney disease, chronic obstructive pulmonary disease, sleep apnea, depression, anemia, and thyroid disease), and total number of co-existing cardiovascular conditions (coronary artery disease, peripheral artery disease, atrial fibrillation, valvular heart disease, pulmonary hypertension, and stroke / transient ischemic attack). As with the main analysis, mortality models were adjusted for age, race, sex, year of admission, and hospital of admission. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, USA) and two-sided P-values < 0.05 were considered statistically significant.
Results
From 2005 – 2014, a total of 22,805 hospitalizations were sampled among patients identified as white or black. Of these, 8914 were classified as definite or probable acute decompensated HF. Echocardiography was increasingly utilized from 2005–2014, for both sexes (P for annual trends <0.0001 for both, P for interaction by sex = 0.8); Supplemental Figure 1. Patients lacking echocardiography had a higher burden of comorbidities; however, the temporal changes in annual comorbidity prevalence paralleled those of patients with echocardiography, with no significant difference in comparisons of temporal trends. (Supplemental Table 1). We excluded 461 hospitalizations due to missing 28-day or 1-year mortality outcomes. Of the remaining 8453 patients classified with acute decompensated HF, 5460 (65%) had available ejection fractions abstracted from in-hospital echocardiography reports and were included in our analysis.
After applying the sampling weights, the 5460 patients with available echocardiography corresponded to 24,937 weighted hospitalizations for acute decompensated HF. All subsequent results are weighted by the sampling fraction. Overall, 53% of our study population was female, 68% were white, and the mean age was 75 years. Approximately half were classified with HFrEF (53%), while 47% were classified as HFpEF. Patients with HFpEF (vs. HFrEF) were older and more often white. Over time, the proportion of women increased among patients with HFrEF but decreased among those HFpEF, while other demographic distributions were largely unchanged (Supplemental Figure 2).
Overall comorbidity burden in women and men with HFpEF vs. HFrEF
A total of 16,594 of the 21,866 patients (76%) hospitalized from 2005–2013 had all 15 comorbidities of interest abstracted from the medical record. Serum creatinine was not collected in 2014; abstractions for other comorbidities were missing at random. The average number of comorbidities was higher for patients with HFpEF vs. HFrEF, both for women (5.53 vs. 4.94, P < 0.0001) and men (5.20 vs. 4.82, P = <0.0001). Among women, there was a significant temporal increase in the overall burden of co-morbidities, both for patients with HFpEF (5.17 in 2005–2009 to 5.87 in 2010–2013, P for annual trend <0.0001) and HFrEF (4.78 in 2005–2009 to 5.14 in 2010–2013, P for annual trend <0.0001). A similar pattern was observed in men, with a modest, statistically significant increase in the average number of comorbidities over time for patients with HFpEF (4.94 in 2005–2009 and 5.45 in 2010–2013, P for annual trend <0.0001) as well as HFrEF (4.62 in 2005–2009 and 5.06 in 2010–2013, P for annual trend <0.0001).
Temporal trends in the prevalence of individual comorbidities in women with acute decompensated HFpEF vs. HFrEF
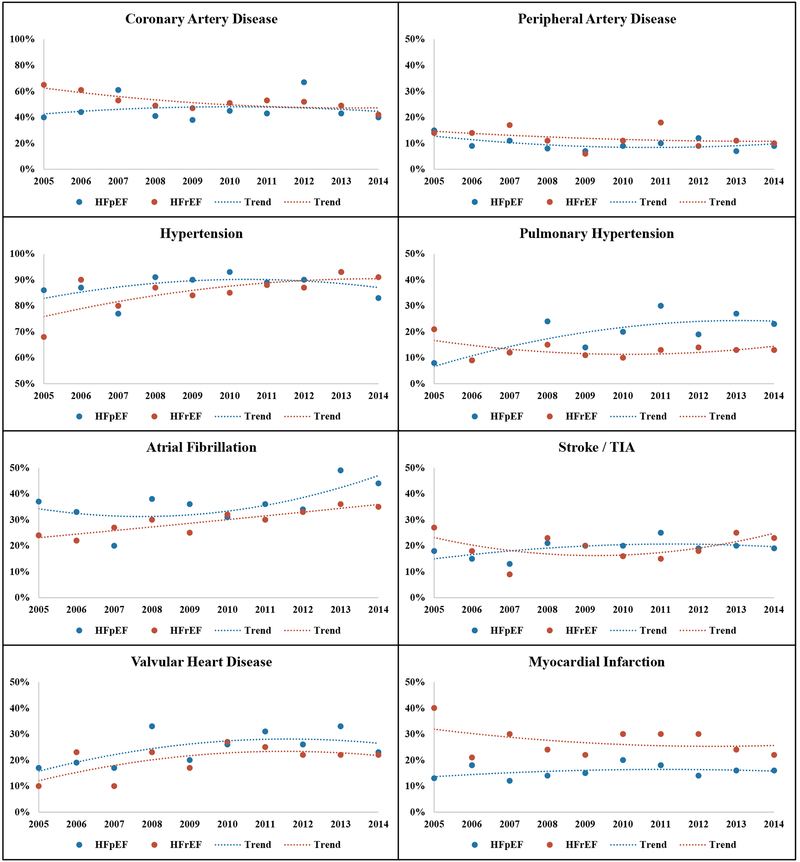
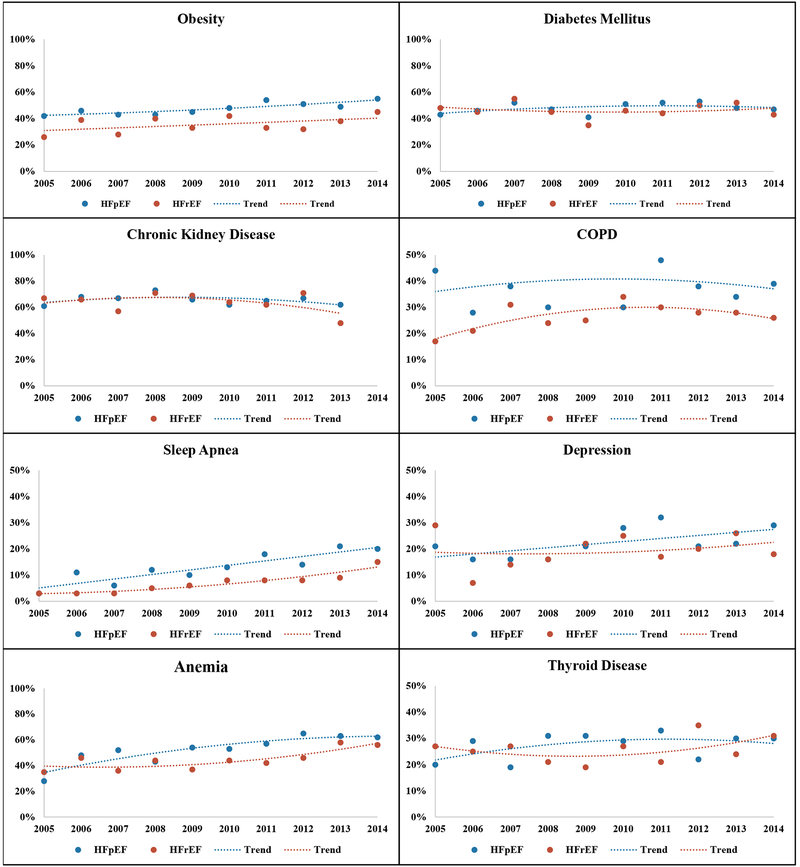
When aggregated across 2005–2014, women hospitalized with HFpEF had a lower prevalence of coronary artery disease than those with HFrEF, but a higher prevalence of other comorbidities such as atrial fibrillation, stroke/TIA, valvular heart disease, pulmonary hypertension, obesity, COPD/bronchitis, sleep apnea, and anemia (Table 1). Across the study period, the annual prevalence of atrial fibrillation, valvular heart disease, and sleep apnea increased for both HF types, yielding parallel trends with statistical comparisons suggesting no interaction by HF type (Table 1, Figures 1 and 2). The annual prevalence of coronary artery disease and hypertension declined over time for women with HFrEF, while remaining stable for those with HFpEF. Obesity, pulmonary hypertension, and depression became increasingly more prevalent for women with HFpEF with no significant change in annual prevalence among those with HFrEF; however, comparisons of temporal trends for women with HFpEF vs. HFrEF differed significantly only for pulmonary hypertension. The annual prevalence of other comorbidities such as peripheral artery disease, stroke/TIA, diabetes, CKD, COPD, thyroid disorders did not significantly change over time for women with either HF subtypes (Table 1, Figures 1 and 2)
Table 1:
Demographics and clinical characteristics of female patients hospitalized with acute decompensated heart failure, stratified by heart failure with preserved vs. reduced ejection fraction and by half-decade in the community surveillance component of the Atherosclerosis Risk in Communities Study, 2005–2014.
| Characteristic | HFpEF | HFrEF | HFpEF vs. HFrEF | |||||
|---|---|---|---|---|---|---|---|---|
|
2005–2009 N=555 N=2769* |
2010–2014 N=944 N=4700* |
Annual Trend P-value† |
2005–2009 N=511 N=2525* |
2010–2014 N=650 N=3106* |
Annual Trend P-value† |
Comparison (Aggregate) P-value‡ |
Comparison (Trends) P-value§ |
|
| Demographics | ||||||||
| Age (± SEM) | 77 ± 0.5 | 78 ± 0.4 | 0.003 | 76 ± 0.5 | 76 ± 0.5 | 0.4 | ||
| White | 2032 (73%) | 3256 (69%) | 0.0001 | 1630 (65%) | 1865 (60%) | 0.0009 | <0.0001 | 0.8 |
| Cardiovascular | ||||||||
| Coronary artery disease | 1198 (43%) | 1933 (41%) | 0.2 | 1371 (54%) | 1540 (50%) | 0.003 | <0.0001 | 0.2 |
| Myocardial infarction | 405 (15%) | 770 (16%) | 0.7 | 673 (27%) | 839 (27%) | 0.2 | <0.0001 | 0.3 |
| Peripheral artery disease | 258 (9%) | 454 (10%) | 0.5 | 301 (12%) | 357 (11%) | 0.2 | 0.1 | 0.7 |
| Hypertension | 2414 (87%) | 4213 (90%) | 0.4 | 2075 (82%) | 2760 (89%) | <0.0001 | 0.06 | 0.07 |
| Atrial fibrillation | 934 (34%) | 1834 (39%) | 0.003 | 647 (26%) | 1024 (33%) | 0.007 | 0.0004 | 0.9 |
| Stroke / TIA | 499 (18%) | 965 (21%) | 0.3 | 495 (20%) | 602 (19%) | 0.6 | 0.9 | 0.8 |
| Valvular heart disease | 612 (22%) | 1304 (28%) | 0.03 | 431 (17%) | 725 (23%) | 0.04 | 0.006 | 0.9 |
| Pulmonary hypertension | 400 (14%) | 1090 (23%) | <0.0001 | 336 (13%) | 391 (13%) | 0.7 | <0.0001 | 0.007 |
| Non-Cardiovascular | ||||||||
| Obesity | 1041 (44%) | 2167 (51%) | 0.02 | 744 (34%) | 1057 (38%) | 0.09 | <0.0001 | 0.7 |
| Diabetes mellitus | 1258 (45%) | 2356 (50%) | 0.3 | 1134 (45%) | 1458 (47%) | 0.9 | 0.3 | 0.6 |
| Chronic kidney disease | 1870 (68%) | 1934 (65%) | 0.8 | 1656 (66%) | 1274 (64%) | 0.8 | 0.8 | 1.0 |
| Chronic bronchitis / COPD | 926 (33%) | 1782 (38%) | 0.3 | 596 (24%) | 899 (29%) | 0.1 | <0.0001 | 0.6 |
| Sleep apnea | 245 (9%) | 806 (17%) | <0.0001 | 107 (4%) | 292 (9%) | 0.0002 | <0.0001 | 0.6 |
| Depression | 497 (18%) | 1217 (26%) | 0.02 | 446 (18%) | 652 (21%) | 0.2 | 0.06 | 0.4 |
| Anemia | 1248 (46%) | 2833 (61%) | 0.9 | 986 (39%) | 1509 (49%) | 0.0004 | <0.0001 | 0.1 |
| Thyroid disease | 761 (27%) | 1334 (28%) | 0.4 | 587 (23%) | 851 (27%) | 0.3 | 0.2 | 0.8 |
Weighted sample sizes
P-values for temporal increase or decrease in annual prevalence assessed by logistic regression, regressing on year of admission and using the Cochrane-Armitage test for trend. P-values for temporal increase or decrease in annual mean values tested by linear regression regressing on year of admission.
P-values for aggregate comparisons between HFpEF vs. HFrEF analyzed by aggregating data across 2005–2014, with mean values tested by least square means from linear regression, and prevalence values tested by Rao-Scott χ2 tests.
P-values comparing differences in temporal comorbidity or demographic trends between HFpEF vs. HFrEF tested by the linear or logistic regression, regressing on heart failure type and year of admission and testing the multiplicative interaction of heart failure type by year of admission
Definition of coronary artery disease includes myocardial infarction. Obesity defined by a BMI ≥30 m/kg2 and limited to patients with available height and weight data (11% missing)
Chronic kidney disease defined by receipt of dialysis or an eGFR <45 mL/min/1.73m2 in patients with available serum creatinine assessments (22% missing, no creatinine abstractions performed in 2014)
Figure 1:
Annual prevalence of cardiovascular comorbidities among women hospitalized with acute decompensated heart failure in 2005–2014, stratified by heart failure with preserved vs. reduced ejection fraction. The community surveillance component of the Atherosclerosis Risk in Communities Study (ARIC).
Figure 2:
Annual prevalence of non-cardiovascular comorbidities among women hospitalized with acute decompensated heart failure in 2005–2014, stratified by heart failure with preserved vs. reduced ejection fraction. The community surveillance component of the Atherosclerosis Risk in Communities Study (ARIC).
Temporal trends in the prevalence of individual comorbidities in men with acute decompensated HFpEF vs. HFrEF
Men hospitalized with HFpEF had a lower prevalence of coronary artery disease than those with HFrEF, when aggregated across 2005–2014. In contrast, the prevalence of obesity, COPD/bronchitis, sleep apnea, and anemia were significantly higher in patients with HFpEF. There was no significant difference in the aggregate prevalence of other comorbidities such as hypertension, diabetes, atrial fibrillation, chronic kidney disease, or depression among men with HFpEF vs. HFrEF (Table 2). Across the study period, the annual prevalence of atrial fibrillation, sleep apnea, and anemia significantly increased for both HF types, while the annual prevalence of coronary artery disease decreased (Table 2, Supplemental Figures 3 and 4). Although the annual prevalence of hypertension and obesity increased only for men with HFrEF, comparisons of temporal trends did not suggest significant interaction by HF type. In contrast, the annual prevalence of depression and valvular heart disease increased significantly more over time for men with HFpEF relative to those with HFrEF, with significant interaction by HF type. The annual prevalence of other comorbidities such as stroke/TIA, diabetes, CKD, and thyroid disorders did not change over time among men with either HF subtype.
Table 2:
Demographics and clinical characteristics of male patients hospitalized with acute decompensated heart failure, stratified by heart failure with preserved vs. reduced ejection fraction and by half-decade in the community surveillance component of the Atherosclerosis Risk in Communities Study, 2005–2014.
| Characteristic | HFpEF | HFrEF | HFpEF vs. HFrEF | |||||
|---|---|---|---|---|---|---|---|---|
|
2005–2009 N=348 N=1509* |
2010–2014 N=629 N=2635* |
Annual Trend P-value† |
2005–2009 N=678 N=2915* |
2010–2014 N=1145 N=4777* |
Annual Trend P-value† |
Comparison (Aggregate) P-value‡ |
Comparison (Trends) P-value§ |
|
| Demographics | ||||||||
| Age (± SEM) | 75 ± 0.6 | 75 ± 0.5 | 0.8 | 73 ± 0.5 | 74 ± 0.4 | 0.08 | 0.0002 | 0.6 |
| White | 1092 (72%) | 1922 (73%) | 0.8 | 1948 (67%) | 3180 (67%) | 0.1 | 0.0009 | 0.7 |
| Cardiovascular | ||||||||
| Coronary artery disease | 888 (59%) | 1385 (53%) | 0.003 | 1986 (68%) | 3068 (64%) | 0.05 | <0.0001 | 0.4 |
| Myocardial infarction | 324 (21%) | 549 (20%) | 0.3 | 1067 (36%) | 1561 (33%) | 0.08 | <0.0001 | 0.9 |
| Peripheral artery disease | 212 (14%) | 329 (13%) | 0.4 | 487 (17%) | 641 (13%) | 0.06 | 0.3 | 0.8 |
| Hypertension | 1316 (87%) | 2308 (88%) | 0.5 | 2325 (80%) | 4191 (88%) | <0.0001 | 0.1 | 0.1 |
| Atrial fibrillation | 490 (32%) | 1136 (43%) | 0.009 | 905 (31%) | 1813 (38%) | 0.05 | 0.08 | 0.4 |
| Stroke / TIA | 313 (21%) | 482 (18%) | 0.7 | 524 (18%) | 990 (21%) | 0.2 | 0.8 | 0.3 |
| Valvular heart disease | 278 (18%) | 705 (27%) | 0.006 | 589 (20%) | 1064 (22%) | 0.7 | 0.2 | 0.09 |
| Pulmonary hypertension | 164 (11%) | 430 (16%) | 0.06 | 325 (11%) | 562 (12%) | 0.6 | 0.07 | 0.3 |
| Non-Cardiovascular | ||||||||
| Obesity | 550 (40%) | 1062 (44%) | 0.1 | 617 (24%) | 1308 (30%) | 0.007 | <0.0001 | 0.7 |
| Diabetes mellitus | 694 (46%) | 1207 (46%) | 0.6 | 1336 (46%) | 2297 (48%) | 0.6 | 0.5 | 0.8 |
| Chronic kidney disease | 923 (62%) | 964 (60%) | 0.08 | 1703 (58%) | 1605 (57%) | 0.5 | 0.2 | 0.3 |
| Chronic bronchitis / COPD | 491 (33%) | 990 (38%) | 0.01 | 875 (30%) | 1490 (31%) | 0.6 | 0.02 | 0.2 |
| Sleep apnea | 188 (12%) | 519 (20%) | 0.001 | 195 (7%) | 601 (13%) | 0.0004 | <0.0001 | 0.9 |
| Depression | 108 (7%) | 428 (16%) | <0.0001 | 285 (10%) | 594 (12%) | 0.2 | 0.3 | 0.05 |
| Anemia | 632 (42%) | 1363 (52%) | 0.003 | 979 (34%) | 1980 (42%) | 0.001 | <0.0001 | 0.8 |
| Thyroid disease | 158 (10%) | 408 (15%) | 0.3 | 307 (11%) | 623 (13%) | 0.1 | 0.3 | 0.9 |
Weighted sample sizes
P-values for temporal increase or decrease in annual prevalence assessed by logistic regression, regressing on year of admission and using the Cochrane-Armitage test for trend. P-values for temporal increase or decrease in annual mean values tested by linear regression regressing on year of admission.
P-values for aggregate comparisons between HFpEF vs. HFrEF analyzed by aggregating data across 2005–2014, with mean values tested by least square means from linear regression, and prevalence values tested by Rao-Scott χ2 tests.
P-values comparing differences in temporal comorbidity or demographic trends between HFpEF vs. HFrEF tested by the linear or logistic regression, regressing on heart failure type and year of admission and testing the multiplicative interaction of heart failure type by year of admission
Definition of coronary artery disease includes myocardial infarction. Obesity defined by a BMI ≥30 m/kg2 and limited to patients with available height and weight data (9% missing)
Chronic kidney disease defined by receipt of dialysis or an eGFR <45 mL/min/1.73m2 in patients with available serum creatinine assessments (25% missing, no creatinine abstractions performed in 2014)
Coronary Revascularization and Therapies
Patients with HFrEF more often had history of coronary revascularization by coronary artery bypass graft surgery or percutaneous coronary intervention than patients with HFpEF, both in the overall sample and when stratified by sex (Supplemental Figure 5). The annual proportion of patients with history of percutaneous coronary intervention increased over time for women (P for annual trend = 0.005) but remained stable for men (P for annual trend = 0.7). When stratified by HF type, the annual proportion of patients with history of percutaneous coronary intervention increased with marginal significance (HFpEF: P for annual trend = 0.1, HFrEF: P for annual trend = 0.06). From 2005–2014, there was an overall increase in administration of beta blockers (P for annual trend = 0.01), which did not differ by HF type (P for interaction = 0.8). Conversely, there was an overall decrease in administration of ACEi / ARB (P for annual trend <0.0001), which was not modified by HF type (P for interaction = 0.8). Similar trends were also observed when stratified by sex (Supplemental Figure 6).
Outcomes
The overall mean length of hospital stay was 8 days. A total of 1635 patients died while in-hospital, 2576 died within 28 days of hospitalization and 7,777 died within a year. Women hospitalized with HFrEF had longer hospitalizations than those with HFpEF (9 vs. 8 days, P=0.03) and a higher incidence of in-hospital (8% vs. 5%; P=0.01), 28-day (12% vs. 9%; P=0.05), and 1-year mortality (32% vs. 29%; P=0.08). Mean length of stay was comparable for men with HFrEF vs. HFpEF (9 days each; P=0.6), as was in-hospital mortality (7% vs. 5%; P=0.09) and death within 28-days (10% for both; P=0.7) and 1-year (32% for both; P=0.9).
Association between comorbidity burden and outcomes in patients with acute decompensated HFpEF vs. HFrEF
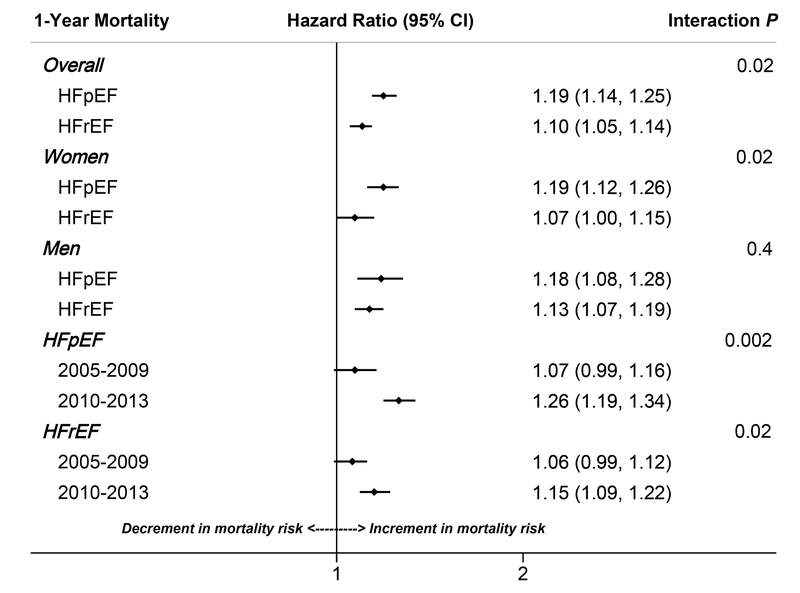
Higher comorbidity burden was significantly associated with 28-day mortality among patients with HFpEF (HR = 1.19; 95% CI: 1.10 – 1.29) but not HFrEF (HR = 1.07; 95% CI: 0.99 – 1.16; P for interaction by HF type = 0.1). This pattern of association with 28-day mortality was consistent across both sexes and throughout the study period (Supplemental Table 2). Within 1 year of hospitalization, higher number of comorbidities was significantly associated with greater adjusted risk of mortality for both HF subtypes, with a stronger association noted for HFpEF vs. HFrEF. With each additional comorbidity, the 1-year mortality risk increased by 19% for patients with HFpEF (HR = 1.19; 95% CI: 1.14 – 1.25), and by 10% for patients with HFrEF [(HR = 1.10; 95% CI: 1.05 – 1.14); P for interaction by HF type = 0.02]. Among women, the association between increasing comorbidity burden and risk of 1-year mortality was stronger with HFpEF [HR (95% CI) per 1 higher comorbidity = 1.19 (1.12 – 1.26)], compared to HFrEF = [HR = 1.07 (1.00 – 1.15)], P for interaction by HF type = 0.02. In contrast, the association was comparable among men, irrespective of HF type [HR (95% CI) per 1 higher comorbidity in patients with HFpEF = 1.18 (1.08 – 1.28) vs. HR = 1.13 (1.07 – 1.19) among patients with HFrEF], P for interaction by HF type = 0.4, (Figure 3). Across the study period, the 1-year risk of mortality associated with co-morbidity burden increased significantly for patients with HFpEF (HR of mortality per 1 higher comorbidity = 1.07 (95% CI: 0.99 – 1.16) in 2005–2009 vs. 1.26 (95% CI: 1.19 – 1.34) in 2010–2013); P for interaction by half-decade = 0.002. A similar association was observed in patients with HFrEF (HR of mortality per 1 higher comorbidity = 1.06 (95% CI: 0.99 – 1.12) in 2005–2009 vs. 1.15 (95% CI: 1.09 – 1.22) in 2010–2013; P for interaction by half-decade = 0.02).
Figure 3:
Increment in 1-year mortality hazard ratio per 1-higher comorbidity among patients hospitalized with acute decompensated heart failure, stratified by heart failure type. The community surveillance component of the Atherosclerosis Risk in Communities (ARIC) study, 2005–2013*
*Footnote: models adjusted for age, race, sex, year of admission, and hospital of admission
HFpEF = heart failure with preserved ejection fraction, HFrEF = heart failure with reduced ejection fraction
Individual comorbidities and mortality risk
Associations between individual comorbidities and 1-year all-cause mortality among patients with HFpEF and HFrEF are shown in Table 3. The presence of comorbidities such as peripheral artery disease, atrial fibrillation, stroke/TIA, valvular heart disease, pulmonary hypertension, CKD, COPD, depression, anemia, and thyroid disorder was associated with higher hazards of 1-year mortality, after adjustment for age, sex, race, year of admission and hospital of admission. Mortality hazards were largely comparable by HF type, with the exception of atrial fibrillation and sleep apnea, which were associated with stronger risk of death in patients with HFpEF. When stratified by sex, individual comorbidities were associated with similar mortality risks for HFpEF and HFrEF among women. However, among men, significant modification by HF type was observed, with atrial fibrillation and valvular heart disease associated with stronger mortality risks in male patients HFpEF compared to HFrEF (Supplemental Table 3).
Table 3:
Hazard ratios of 1-year all-cause mortality associated with individual comorbidities among patients hospitalized with acute decompensated heart failure, stratified by heart failure type. The community surveillance component of the Atherosclerosis Risk in Communities study, 2005–2014*.
| Comorbidity | HFpEF | HFrEF | Interaction by HF type |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | P-value | |
| Coronary artery disease | 1.13 (0.96 – 1.34) | 0.97 (0.84 – 1.12) | 0.1 |
| Myocardial infarction | 1.05 (0.86 – 1.29) | 1.02 (0.88 – 1.19) | 0.7 |
| Peripheral artery disease | 1.33 (1.06 – 1.68) | 1.43 (1.19 – 1.72) | 0.7 |
| Hypertension | 0.85 (0.66 – 1.04) | 1.11 (0.89 – 1.38) | 0.08 |
| Atrial fibrillation | 1.34 (1.14 – 1.57) | 0.97 (0.83 – 1.13) | 0.003 |
| Stroke / TIA | 1.40 (1.16 – 1.69) | 1.13 (0.95 – 1.34) | 0.1 |
| Valvular heart disease | 1.27 (1.06 – 1.53) | 1.19 (1.01 – 1.40) | 0.6 |
| Pulmonary hypertension | 1.42 (1.17 – 1.72) | 1.26 (1.03 – 1.54) | 0.5 |
| Obesity (BMI ≥30) | 0.88 (0.73 – 1.06) | 0.90 (0.76 – 1.07) | 1.0 |
| Obesity categories | 0.2 | ||
| BMI <30 | ref. | ref. | |
| BMI 30–35 | 0.76 (0.60 – 0.98) | 0.94 (0.77 – 1.16) | |
| BMI (BMI ≥35) | 1.00 (0.80 – 1.24) | 0.85 (0.66 – 1.09) | |
| Diabetes mellitus | 1.09 (0.92 – 1.29) | 1.07 (0.93 – 1.23) | 0.9 |
| Chronic kidney disease | 1.58 (1.29 – 1.95) | 1.69 (1.41 – 2.02) | 0.6 |
| Chronic bronchitis / COPD | 1.42 (1.20 – 1.68) | 1.28 (1.09 – 1.49) | 0.3 |
| Sleep apnea | 1.19 (0.95 – 1.50) | 0.89 (0.68 – 1.16) | 0.03 |
| Depression | 1.51 (1.25 – 1.84) | 1.38 (1.14 – 1.68) | 0.6 |
| Anemia | 1.70 (1.43 – 2.01) | 1.60 (1.40 – 1.84) | 0.8 |
| Thyroid disease | 1.37 (1.14 – 1.66) | 1.12 (0.93 – 1.36) | 0.2 |
Footnote: models adjusted for age, race, sex, year of admission, and hospital of admission. Definition of coronary artery disease include myocardial infarction. Chronic kidney disease not abstracted in 2014.
Abbreviations: HFpEF = heart failure with preserved ejection fraction, HFrEF = heart failure with reduced ejection, HF = heart failure
Trends in primary HF risk factor burden and co-existing cardiovascular conditions
On average, patients with HFpEF had a higher number of primary HF risk factors (out of 9 possible comorbidities) than patients with HFrEF (3.85 vs. 3.29; P < 0.0001), but the mean number of co-existing cardiovascular conditions (out of 6 possible comorbidities) was similar (1.58 vs. 1.59; P = 0.7). Over time, the mean number of primary HF risk factors increased from 2005–2009 to 2010–2013, both for HFpEF (3.61 to 4.07; P for annual trend <0.0001) and for HFrEF (3.14 to 3.47; P for annual trend <0.0001). The average number of co-existing cardiovascular conditions also increased from 2005–2009 to 2010–2014 for patients with HFpEF (1.46 to 1.64; P for annual trend = 0.005) but did not significantly change for those with HFrEF (1.54 to 1.62; P for annual trend = 0.6), Supplemental Figure 7. Burden of primary HF risk factors was associated with increased 1-year mortality risk, both for HFpEF (HR per 1 higher risk factor = 1.23; 95% CI: 1.15 – 1.31) and for HFrEF (HR per 1 higher risk factor = 1.14; 95% CI: 1.08 – 1.21); P for interaction by HF type = 0.2. In contrast, the association between burden of cardiovascular manifestations and 1-year mortality differed significantly by HF type, yielding a HR per 1 higher cardiovascular manifestation of 1.20 (95% CI: 1.13 – 1.28) for patients with HFpEF vs. 1.08 (95% CI: 1.02 – 1.15) for patients with HFrEF; P for interaction by HF type = 0.01.
Discussion
In this community-based surveillance of approximately 25,000 weighted hospitalizations for acute decompensated HF spanning 2005–2014, there were several important findings. First, the burden of comorbidities has increased over time among patients with HFpEF and HFrEF, with a higher burden noted among those with HFpEF. Second, a greater burden of comorbidities is associated with significantly higher risk of 1-year mortality for both HFpEF and HFrEF, with significant sex differences. The associations between co-morbidity burden and risk of 1-year mortality was stronger for women with HFpEF vs. HFrEF and comparable across both HF subtypes in men. Third, the 1-year mortality risk associated with comorbidity burden increased significantly over time, both for patients with HFrEF and HFpEF. Finally, mortality risks associated with individual co-morbidities were comparable across HF subtypes, with the exception of atrial fibrillation and sleep apnea which were more strongly associated with mortality in patients with HFpEF. Taken together, our study findings provide insights into the distinct temporal trends and prognostic implications of overall and individual comorbidities in patients with acute decompensated HFpEF and HFrEF.
Prior studies have demonstrated a high burden of comorbidities in patients with chronic stable HF.6, 13, 14, 16 Consistent with our observations, an analysis from a national ambulatory cohort of Veterans with HF13 demonstrated higher burden of comorbidities in patients with HFpEF vs. HFrEF. More recently, Sharma et al.8 observed a temporal increase in the burden of comorbidities among patients hospitalized with acute HF in the Get with The Guidelines®-Heart Failure (GWTG-HF) cohort. Similarly, a recent population-based study in the United Kingdom demonstrated that the number of comorbidities associated with HF has increased steadily over time, from a mean of 3.4±1.9 in 2002 to 5.4±2.5 in 2014.7 Our study adds to these observations by evaluating contemporary trends stratified both by sex and by HF type. We observed a significant temporal increase in the comorbidity burden and its associated mortality risk, both for patients with HFpEF and HFrEF; findings which highlight the growing complexity of the clinical management of patients with acute decompensated HF over time.
When analyzed individually, a significant temporal decline in the prevalence of CAD was observed among men and women with both HF subtypes. Although CAD and prior myocardial infarction are well-established risk factors for HFrEF,17–19 recent studies have also implicated CAD as a significant risk factor for HFpEF, with a comparable association to HFrEF.18, 20 Consistent with our observations, prior studies have reported the prevalence of CAD in patients with HFpEF in the range of 40–50%.9, 21 The decline in CAD burden noted in our study is consistent with an overall decline in the atherosclerotic cardiovascular diseases in the general population.22 We also noted a concomitant increase in the burden of non-atherosclerotic comorbidities such as atrial fibrillation, sleep apnea, and anemia across both HF subtypes. This highlights the evolving epidemiology of HF in the contemporary era with a gradual but significant shift from an ischemic etiology HF to multimorbidity-related HF.
Several factors may underlie the increasing burden of comorbidities in patients with acute decompensated HF. These include the aging population, greater use of screening and diagnostic tests for co-morbid conditions in the clinical practice, greater physician awareness of the non-cardiovascular comorbidities that play an important role in clinical decompensation of patients with HF, particularly HFpEF, changes in administrative coding practices with expansion of secondary diagnoses codes by Medicare,23 and evolution in the risk factors for HF, particularly HFpEF. Future studies are needed to better understand the extent to which these factors contribute to the growing multimorbidity in acute decompensated HFpEF and HFrEF.
We also observed that greater burden of comorbidities was associated with higher risk of mortality in patients with acute decompensated HF. This is consistent with the prior literature in patients with chronic stable HF.13, 16, 24, 25 More recently, Sharma et al.8 demonstrated a significant association between the number of comorbidities and risk of mortality among hospitalized patients with acute decompensated HF from the GWTG-HF registry. Similar findings were also observed in a recent secondary analysis of the ASCEND trial.26 Findings from the present study add to the existing literature by demonstrating distinct temporal patterns in the association between comorbidity burden and risk of 1-year mortality among patients with acute decompensated HF, with a stronger association noted in the latter part of the study period. Furthermore, the association between higher co-morbidity burden and 1-year mortality risk was modified by HF type with a stronger association observed among patients with HFpEF than HFrEF. These temporal trends highlight the worsening complexity of acute decompensated HF patients and the detrimental long-term prognostic effects of increasing multimorbidity, particularly among patients with HFpEF who lack effective therapies to improve clinical outcomes.9
We also observed that the association between co-morbidity burden and 1-year mortality risk for patients with HFpEF vs. HFrEF differed across sexes. Among women, increasing number of co-morbidities was more strongly associated with risk of 1-year mortality for HFpEF vs. HFrEF. In contrast, among men the risk of mortality associated with increasing co-morbidity burden was comparable for both HF subtypes. These sex differences in the prognostic implications of co-morbidity burden across HF subtypes may be related to several factors. First, we observed important differences in the prevalence of specific co-morbidities among patients with HFpEF vs. HFrEF across sexes. The prevalence of the majority of co-morbidities (11 out of 16) assessed in our study were comparable among men with HFpEF vs. HFrEF. In contrast, women had more prominent differences in the prevalence of specific co-morbidities across the two HF subtypes. Particularly, the burden of prognostically relevant conditions such as atrial fibrillation, pulmonary hypertension, and valvular heart disease was higher in women with HFpEF vs. HFrEF and comparable among men across the two HF subtypes. Previous studies have reported clinically meaningful sex differences in the pathophysiology and epidemiology of HF, particularly HFpEF, which may modify the observed adverse effects of increasing co-morbidity burden. 27–30 Future studies are needed to better understand the biological mechanisms that may drive the observed sex differences in mortality risk associated with HFpEF vs. HFrEF.
In addition to the overall co-morbidity burden, the present study also adds to the existing literature by evaluating the association of specific co-morbidities with risk of 1-year mortality among patients with HFpEF and HFrEF. Consistent with prior literature, co-morbidities such as pulmonary hypertension, peripheral arterial disease, valvular disease, chronic kidney disease, depression, and anemia were significantly associated with higher risk of mortality across both HF subtypes.13, 14, 25, 31 An obesity paradox was also observed, with lower risk of mortality among obese vs. overweight patients, particularly for obese class I patients with HFpEF. It is noteworthy that atrial fibrillation and sleep apnea were associated with higher risk of mortality in patients with HFpEF but not HFrEF. Consistent with our observation, prior observations from European cohorts of patients with HF (HF long-term registry of the European Society of Cardiology) have also demonstrated a stronger association between atrial fibrillation and risk of mortality among patients with HFpEF vs. HFrEF.32, 33 Treatment of sleep apnea has also been shown to have a more favorable effect on clinical outcomes in patients with HFpEF vs. HFrEF.34 Taken together, these findings highlight the prognostic significance of these specific co-morbidities among patients hospitalized with acute decompensated HFpEF and HFrEF.
Our observations from the ARIC study community surveillance have important clinical and public health implications. The overall prevalence of HF, particularly HFpEF, continues to increase in the community.12 An important aspect of this is the accompanied and increasing co-morbidity burden of the aging population. Findings from our study provide important insight into the temporal trends in the prevalent co-morbidities and their impact on long-term outcomes. Specifically, we observed a shift from ischemic etiology HF to multimorbidity HF over time, particularly among patients with HFpEF. The patterns of risk factor and co-morbidity burden observed in our study supports the notion that clinical HFpEF may not be related to a single cardiac disease but is rather a manifestation related to chronic microvascular dysfunction across multiple organ systems.35 This highlights the importance of considering individual and overall comorbidity burden in guiding the management decisions for HF. Along these lines, aggressive management of co-morbid conditions such as anemia36, sleep apnea34, atrial fibrillation37, and obesity38 have been shown to reduce the burden of HF hospitalization and mortality in small RCTs and observational studies. Moreover, holistic approaches targeting multi-morbidity burden have also been proposed to improve outcomes of patients with HF, particularly in older patients with higher co-morbidity burden.39–41
The ARIC study community surveillance has several important strengths. These include the large sample of patients with clinically adjudicated acute decompensated HF, hospitalizations spanning a 10-year period in 4 geographically diverse regions of the US, standardized medical record abstractions of clinical data and prevalent comorbidities by certified abstractors, and ascertainment of mortality outcomes from the national death index. Certain limitations to our study are also noteworthy. First, longitudinal outcomes other than vital status were not available in the in the community surveillance component of the ARIC study. As a result, we cannot examine the association of comorbidity burden with future clinical events such as HF hospitalization or cause-specific deaths during follow-up. Second, the ARIC study community surveillance excludes individuals <55 years of age, limiting generalizability to younger patients. However, prior studies from Swedish HF registry have demonstrated that HF events in younger individuals constitute a very small fraction of the overall HF burden in the community (~4% of all HF cases).42 Similarly, in the US, the proportion of patients hospitalized with HF who are less than 45 years of age is 3%.43 Consequently, the inclusion of younger individuals with HF would only have a modest impact on the observed temporal trends in co-morbidity burden and its association with risk of mortality among all patients with HF. Third, we were unable to consider outpatients with HF since the focus of the ARIC study community surveillance is hospitalized acute decompensated HF. The diagnosis of clinical HF in the outpatient setting is challenging and not consistently captured in most population studies.44 A future research direction, possibly incorporating the 5% Medicare beneficiaries sample, could evaluate differences in co-morbidity burden among hospitalized vs. outpatient HF patients. Fourth, echocardiographic assessment of ejection fraction was only available in 65% of the study population and thus, the observed trends are prone to selection bias. However, the proportion of patients with available EF data is similar to that reported in other studies. Furthermore, we did not observe any meaningful differences in the temporal trends in co-morbidities among patients with vs. without echocardiographic assessment. Finally, our analysis was limited to data abstracted from the medical record, and we do not have data on some co-morbidities such as cancer. Furthermore, we do not have information concerning the severity and management of comorbidities, limiting our ability to evaluate whether optimal management may modify the mortality risk.
Conclusion
In conclusion, we observed an increasing burden of comorbidities among patients with hospitalized acute decompensated HFpEF and HFrEF over time. The heightened mortality risk associated with higher burden of comorbidities was stronger for patients with HFpEF vs. HFrEF, and in the more recent relative to earlier years of the study surveillance period. Future studies are needed to assess whether optimal management of comorbid conditions over time may lower the risk of adverse outcomes in patients with HF, particularly HFpEF.
Supplementary Material
Clinical Perspective.
What is new
This analysis of the community surveillance component of the ARIC (Atherosclerosis Risk in Communities) study demonstrated a significant increase in the burden of co-morbidities among hospitalized patients with HFpEF as well as HFrEF across both sexes.
Higher number of co-morbidities was associated with higher risk of 1-year mortality, with a stronger association noted among patients with HFpEF vs. HFrEF
The 1-year mortality risk associated with increasing co-morbidity burden has also increased over time.
What are the clinical Implications:
Our study findings demonstrate a shift from ischemic etiology HF to multimorbidity HF over time, particularly among patients with HFpEF.
This highlights the importance of holistic approaches targeting multi-morbidity burden in guiding the management of patients with acute decompensated HF.
Acknowledgments
Drs. Pandey and Caughey conceptualized the study, interpreted the data, and wrote the manuscript. Dr. Caughey performed the statistical analysis. Drs. Vaduganathan, Arora, Qamar, Mentz, Shah, Chang, Russell, and Rosamond revised the manuscript critically. The authors thank the staff and participants of the ARIC study for their important contributions.
Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract numbers (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I).
Conflict of Interests and Disclosures
Dr. Mentz receives research support from the National Institutes of Health (U01HL125511-01A1 and R01AG045551-01A1), Akros, Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Gilead, InnoLife, Luitpold/American Regent, Medtronic, Merck, Novartis and Sanofi; honoraria from Abbott, Amgen, AstraZeneca, Bayer, Boston Scientific, Janssen, Luitpold Pharmaceuticals, Medtronic, Merck, Novartis, and Sanofi; and has served on an advisory board for Amgen, AstraZeneca, Luitpold, Merck, Novartis and Boehringer Ingelheim. Dr. Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541), serves on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, and Boehringer Ingelheim, and participates on clinical endpoint committees for studies sponsored by Novartis and the NIH. Dr. Pandey is supported by the Texas Health Resources Research Scholarship and has served on the advisory board of Roche Diagnostics.
Non-standardized Abbreviations:
- ARIC
Atherosclerosis Risk in Communities
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- EF
Ejection fraction
- ACEi
Angiotensin converting enzyme inhibitors
- ARB
Angiotensin receptor blockers
- COPD
Chronic obstructive pulmonary disease
- TIA
Transient ischemic attack
- CKD
Chronic kidney disease
References:
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG, American Heart Association Advocacy Coordinating C, Council on Arteriosclerosis T, Vascular B, Council on Cardiovascular R, Intervention, Council on Clinical C, Council on E, Prevention and Stroke C. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khera R, Pandey A, Ayers CR, Agusala V, Pruitt SL, Halm EA, Drazner MH, Das SR, de Lemos JA and Berry JD. Contemporary Epidemiology of Heart Failure in Fee-For-Service Medicare Beneficiaries Across Healthcare Settings. Circ Heart Fail. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, Kjeldsen K, Jankowska EA, Atar D, Butler J, Fiuzat M, Zannad F, Pitt B and O’Connor CM. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edelmann F, Stahrenberg R, Gelbrich G, Durstewitz K, Angermann CE, Dungen HD, Scheffold T, Zugck C, Maisch B, Regitz-Zagrosek V, Hasenfuss G, Pieske BM and Wachter R. Contribution of comorbidities to functional impairment is higher in heart failure with preserved than with reduced ejection fraction. Clin Res Cardiol. 2011;100:755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triposkiadis F, Giamouzis G, Parissis J, Starling RC, Boudoulas H, Skoularigis J, Butler J and Filippatos G. Reframing the association and significance of co-morbidities in heart failure. Eur J Heart Fail. 2016;18:744–58. [DOI] [PubMed] [Google Scholar]
- 7.Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV and Rahimi K. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma A, Zhao X, Hammill BG, Hernandez AF, Fonarow GC, Felker GM, Yancy CW, Heidenreich PA, Ezekowitz JA and DeVore AD. Trends in Noncardiovascular Comorbidities Among Patients Hospitalized for Heart Failure: Insights From the Get With The Guidelines-Heart Failure Registry. Circ Heart Fail. 2018;11:e004646. [DOI] [PubMed] [Google Scholar]
- 9.Lam CS, Donal E, Kraigher-Krainer E and Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM and Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, Gottdiener JS, Psaty BM and Vasan RS. Temporal Trends in the Incidence of and Mortality Associated With Heart Failure With Preserved and Reduced Ejection Fraction. JACC Heart Fail. 2018;6:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang PP, Wruck LM, Shahar E, Rossi JS, Loehr LR, Russell SD, Agarwal SK, Konety SH, Rodriguez CJ and Rosamond WD. Trends in Hospitalizations and Survival of Acute Decompensated Heart Failure in Four US Communities (2005–2014): ARIC Study Community Surveillance. Circulation. 2018;138:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH and Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iorio A, Senni M, Barbati G, Greene SJ, Poli S, Zambon E, Di Nora C, Cioffi G, Tarantini L, Gavazzi A, Sinagra G and Di Lenarda A. Prevalence and prognostic impact of noncardiac co-morbidities in heart failure outpatients with preserved and reduced ejection fraction: a community-based study. Eur J Heart Fail. 2018;20:1257–1266. [DOI] [PubMed] [Google Scholar]
- 15.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G and Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R and Wu AW. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–33. [DOI] [PubMed] [Google Scholar]
- 17.Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC and McAlister FA. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53:13–20. [DOI] [PubMed] [Google Scholar]
- 18.Pandey A, Omar W, Ayers C, LaMonte M, Klein L, Allen NB, Kuller LH, Greenland P, Eaton CB, Gottdiener JS, Lloyd-Jones DM and Berry JD. Sex and Race Differences in Lifetime Risk of Heart Failure With Preserved Ejection Fraction and Heart Failure With Reduced Ejection Fraction. Circulation. 2018;137:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D’Agostino RB, Levy D, Kannel WB and Vasan RS. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerber Y, Weston SA, Berardi C, McNallan SM, Jiang R, Redfield MM and Roger VL. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am J Epidemiol. 2013;178:1272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfeffer MA, Shah AM and Borlaug BA. Heart Failure With Preserved Ejection Fraction In Perspective. Circ Res. 2019;124:1598–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV and Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–65. [DOI] [PubMed] [Google Scholar]
- 23.Sukul D, Hoffman GJ, Nuliyalu U, Adler-Milstein JR, Zhang B, Dimick JB and Ryan AM. Association Between Medicare Policy Reforms and Changes in Hospitalized Medicare Beneficiaries’ Severity of Illness. JAMA Netw Open. 2019;2:e193290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahluwalia SC, Gross CP, Chaudhry SI, Ning YM, Leo-Summers L, Van Ness PH and Fried TR. Impact of comorbidity on mortality among older persons with advanced heart failure. J Gen Intern Med. 2012;27:513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ergatoudes C, Schaufelberger M, Andersson B, Pivodic A, Dahlstrom U and Fu M. Noncardiac comorbidities and mortality in patients with heart failure with reduced vs. preserved ejection fraction: a study using the Swedish Heart Failure Registry. Clin Res Cardiol. 2019;108:1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatt A AA, Dunning A, DeVore A, Butler J, Reed S et al. The burden of noncardiac comorbidities and association with clinical outcomes in an acute heart failure trial – insights from ASCEND-HF. Eur J Heart Fail. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beale AL, Meyer P, Marwick TH, Lam CSP and Kaye DM. Sex Differences in Cardiovascular Pathophysiology: Why Women Are Overrepresented in Heart Failure With Preserved Ejection Fraction. Circulation. 2018;138:198–205. [DOI] [PubMed] [Google Scholar]
- 28.Lau ES, Cunningham T, Hardin KM, Liu E, Malhotra R, Nayor M, Lewis GD and Ho JE. Sex Differences in Cardiometabolic Traits and Determinants of Exercise Capacity in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mauricio R, Patel KV, Agusala V, Singh K, Lewis A, Ayers C, Grodin JL, Berry JD and Pandey A. Sex differences in cardiac function, biomarkers and exercise performance in heart failure with preserved ejection fraction: findings from the RELAX trial. Eur J Heart Fail. 2019;21:1476–1479. [DOI] [PubMed] [Google Scholar]
- 30.O’Meara E, Clayton T, McEntegart MB, McMurray JJ, Pina IL, Granger CB, Ostergren J, Michelson EL, Solomon SD, Pocock S, Yusuf S, Swedberg K, Pfeffer MA and Investigators C. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;115:3111–20. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor C, Fiuzat M, Mulder H, Coles A, Ahmad T, Ezekowitz JA, Adams KF, Pina IL, Anstrom KJ, Cooper LS, Mark DB, Whellan DJ, Januzzi JL Jr., Leifer ES and Felker GM. Clinical factors related to morbidity and mortality in high-risk heart failure patients: the GUIDE-IT predictive model and risk score. Eur J Heart Fail. 2019;21:770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP, Parissis J, Laroche C, Piepoli MF, Fonseca C, Mebazaa A, Lund L, Ambrosio GA, Coats AJ, Ferrari R, Ruschitzka F, Maggioni AP and Filippatos G. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19:1574–1585. [DOI] [PubMed] [Google Scholar]
- 33.Zafrir B, Lund LH, Laroche C, Ruschitzka F, Crespo-Leiro MG, Coats AJS, Anker SD, Filippatos G, Seferovic PM, Maggioni AP, De Mora Martin M, Polonski L, Silva-Cardoso J, Amir O and Investigators E-HHL-TR. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: a report from 14 964 patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur Heart J. 2018;39:4277–4284. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor CM, Whellan DJ, Fiuzat M, Punjabi NM, Tasissa G, Anstrom KJ, Benjafield AV, Woehrle H, Blase AB, Lindenfeld J and Oldenburg O. Cardiovascular Outcomes With Minute Ventilation-Targeted Adaptive Servo-Ventilation Therapy in Heart Failure: The CAT-HF Trial. J Am Coll Cardiol. 2017;69:1577–1587. [DOI] [PubMed] [Google Scholar]
- 35.Crea F, Bairey Merz CN, Beltrame JF, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Camici PG and Coronary Vasomotion Disorders International Study G. The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J. 2017;38:473–477. [DOI] [PubMed] [Google Scholar]
- 36.Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM, Tang WHW, LaRue SJ, Redfield MM, Semigran MJ, Givertz MM, Van Buren P, Whellan D, Anstrom KJ, Shah MR, Desvigne-Nickens P, Butler J, Braunwald E and Network NHFCR. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF Randomized Clinical Trial. JAMA. 2017;317:1958–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turagam MK, Garg J, Whang W, Sartori S, Koruth JS, Miller MA, Langan N, Sofi A, Gomes A, Choudry S, Dukkipati SR and Reddy VY. Catheter Ablation of Atrial Fibrillation in Patients With Heart Failure: A Meta-analysis of Randomized Controlled Trials. Ann Intern Med. 2019;170:41–50. [DOI] [PubMed] [Google Scholar]
- 38.Sundstrom J, Bruze G, Ottosson J, Marcus C, Naslund I and Neovius M. Weight Loss and Heart Failure: A Nationwide Study of Gastric Bypass Surgery Versus Intensive Lifestyle Treatment. Circulation. 2017;135:1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forman DE, Maurer MS, Boyd C, Brindis R, Salive ME, Horne FM, Bell SP, Fulmer T, Reuben DB, Zieman S and Rich MW. Multimorbidity in Older Adults With Cardiovascular Disease. J Am Coll Cardiol. 2018;71:2149–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorodeski EZ, Goyal P, Hummel SL, Krishnaswami A, Goodlin SJ, Hart LL, Forman DE, Wenger NK, Kirkpatrick JN, Alexander KP and Geriatric Cardiology Section Leadership Council ACoC. Domain Management Approach to Heart Failure in the Geriatric Patient: Present and Future. J Am Coll Cardiol. 2018;71:1921–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goyal P, Gorodeski EZ, Flint KM, Goldwater DS, Dodson JA, Afilalo J, Maurer MS, Rich MW, Alexander KP and Hummel SL. Perspectives on Implementing a Multidomain Approach to Caring for Older Adults With Heart Failure. J Am Geriatr Soc. 2019;67:2593–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barasa A, Schaufelberger M, Lappas G, Swedberg K, Dellborg M and Rosengren A. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur Heart J. 2014;35:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Dharmarajan K, Wang Y and Krumholz HM. National trends in heart failure hospital stay rates, 2001 to 2009. J Am Coll Cardiol. 2013;61:1078–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roger VL. Epidemiology of heart failure. Circ Res. 2013;113:646–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.