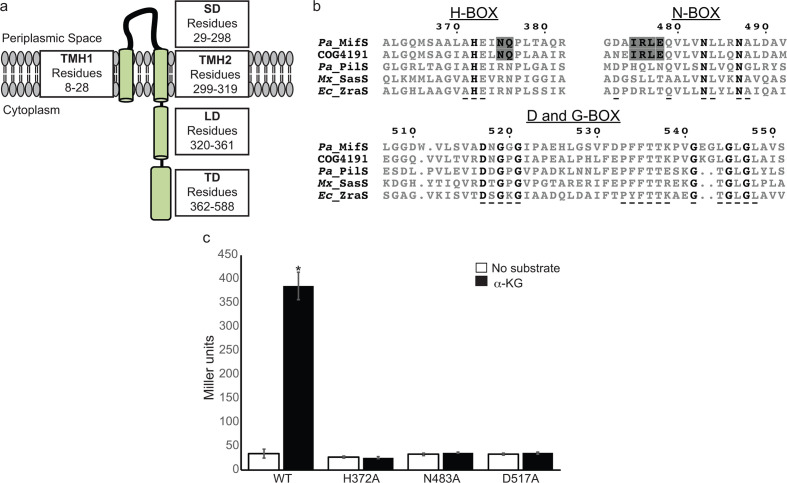
Fig. 1.
MifS is a DctB family transmembrane histidine kinase. (a) Domain architecture of MifS showing a periplasmic sensor domain (SD) flanked by two transmembrane helices (TMH1 and TMH2). TMH2 is followed by a cytoplasmic linker domain (LD) attached to a cytoplasmic transmitter domain (TD). (b) Alignment of the transmitter domain sequences of Pseudomonas aeruginosa MifS, COG4191 consensus sequence representing the DctB family of sensor histidine kinases, and HPK4 family histidine kinases from P. aeruginosa (Pa), Myxococcus xanthus (Mx) and Escherichia coli (Ec). The invariant residues of each transmitter domain conserved sequence box are shown in bold type. Conserved HPK4 sequence motifs are indicated with an underscore (_) . Sequence motifs conserved between MifS and DctB COG4191are highlighted in grey. Alignment was generated using clustal Omega [56] and displayed using ESPript 3.0 [57]. (c) Wild-type MifS, or a MifS variant harbouring a mutation in one of the essential transmitter domain residues (MifSH372A, MifSN483A, or MifSD517A), was expressed in E. coli cells harbouring the mifR gene and the PPA5530::lacZ reporter. Cells were grown in LB media to an optical density at 600 nm (OD600) value of 0.3. They were then challenged with α-KG or no substrate and allowed to grow for an additional 60 min post-induction. Data points represent mean values±the standard deviations (n=3). Analysis of variance was performed by using Dunnett’s post-hoc test (α value of 0.05) to identify significant differences (P<0.0001; marked with an asterisk).